当前位置:
X-MOL 学术
›
J. Comput. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Revised mechanism of carboxylation of ribulose-1,5-biphosphate by rubisco from large scale quantum chemical calculations
Journal of Computational Chemistry ( IF 3.4 ) Pub Date : 2018-05-14 , DOI: 10.1002/jcc.25343 Peter L. Cummins 1 , Babu Kannappan 1 , Jill E. Gready 1
Journal of Computational Chemistry ( IF 3.4 ) Pub Date : 2018-05-14 , DOI: 10.1002/jcc.25343 Peter L. Cummins 1 , Babu Kannappan 1 , Jill E. Gready 1
Affiliation
|
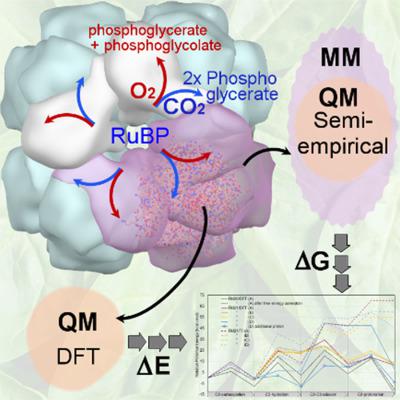
Here, we describe a computational approach for studying enzymes that catalyze complex multi‐step reactions and apply it to Ribulose 1,5‐bisphosphate carboxylase–oxygenase (Rubisco), the enzyme that fixes atmospheric carbon dioxide within photosynthesis. In the 5‐step carboxylase reaction, the substrate Ribulose‐1,5‐bisphosphate (RuBP) first binds Rubisco and undergoes enolization before binding the second substrate, CO2. Hydration of the RuBP.CO2 complex is followed by CC bond scission and stereospecific protonation. However, details of the roles and protonation states of active‐site residues, and sources of protons and water, remain highly speculative. Large‐scale computations on active‐site models provide a means to better understand this complex chemical mechanism. The computational protocol comprises a combination of hybrid semi‐empirical quantum mechanics and molecular mechanics within constrained molecular dynamics simulations, together with constrained gradient minimization calculations using density functional theory. Alternative pathways for hydration of the RuBP.CO2 complex and associated active‐site protonation networks and proton and water sources were investigated. The main findings from analysis of the resulting energetics advocate major revision to existing mechanisms such that: hydration takes place anti to the CO2; both hydration and CC bond scission require early protonation of CO2 in the RuBP.CO2 complex; CC bond scission and stereospecific protonation reactions are concerted and, effectively, there is only one stable intermediate, the C3‐gemdiolate complex. Our main conclusions for interpreting enzyme kinetic results are that the gemdiolate may represent the elusive Michaelis–Menten‐like complex corresponding to the empirical Km (=Kc) with turnover to product via bond scission concerted with stereospecific protonation consistent with the observed catalytic rate. © 2018 Wiley Periodicals, Inc.
中文翻译:

从大规模量子化学计算修正 rubisco 羧化 1,5-二磷酸核酮糖的机理
在这里,我们描述了一种用于研究催化复杂多步反应的酶的计算方法,并将其应用于核酮糖 1,5-二磷酸羧化酶加氧酶 (Rubisco),该酶在光合作用中固定大气二氧化碳。在 5 步羧化酶反应中,底物 1,5-二磷酸核酮糖 (RuBP) 首先结合 Rubisco 并在结合第二个底物 CO2 之前经历烯醇化。RuBP.CO2 复合物的水合之后是 CC 键断裂和立体有择质子化。然而,活性位点残基的作用和质子化状态的细节,以及质子和水的来源,仍然高度推测。对活性位点模型的大规模计算提供了一种更好地理解这种复杂化学机制的方法。计算协议包括约束分子动力学模拟中的混合半经验量子力学和分子力学的组合,以及使用密度泛函理论的约束梯度最小化计算。研究了 RuBP.CO2 复合物和相关活性位点质子化网络以及质子和水源水合的替代途径。对由此产生的能量学分析的主要发现主张对现有机制进行重大修改,例如: 水合发生对抗 CO2;水合和 CC 键断裂都需要在 RuBP.CO2 复合物中进行 CO2 的早期质子化;CC 键断裂和立体定向质子化反应是协调一致的,并且有效地,只有一种稳定的中间体,即 C3-gemdiolate 复合物。我们解释酶动力学结果的主要结论是,gemdiolate 可能代表难以捉摸的 Michaelis-Menten 样复合物,对应于经验 Km(=Kc),通过断键转化为产物,与观察到的催化速率一致的立体定向质子化。© 2018 Wiley Periodicals, Inc.
更新日期:2018-05-14
中文翻译:

从大规模量子化学计算修正 rubisco 羧化 1,5-二磷酸核酮糖的机理
在这里,我们描述了一种用于研究催化复杂多步反应的酶的计算方法,并将其应用于核酮糖 1,5-二磷酸羧化酶加氧酶 (Rubisco),该酶在光合作用中固定大气二氧化碳。在 5 步羧化酶反应中,底物 1,5-二磷酸核酮糖 (RuBP) 首先结合 Rubisco 并在结合第二个底物 CO2 之前经历烯醇化。RuBP.CO2 复合物的水合之后是 CC 键断裂和立体有择质子化。然而,活性位点残基的作用和质子化状态的细节,以及质子和水的来源,仍然高度推测。对活性位点模型的大规模计算提供了一种更好地理解这种复杂化学机制的方法。计算协议包括约束分子动力学模拟中的混合半经验量子力学和分子力学的组合,以及使用密度泛函理论的约束梯度最小化计算。研究了 RuBP.CO2 复合物和相关活性位点质子化网络以及质子和水源水合的替代途径。对由此产生的能量学分析的主要发现主张对现有机制进行重大修改,例如: 水合发生对抗 CO2;水合和 CC 键断裂都需要在 RuBP.CO2 复合物中进行 CO2 的早期质子化;CC 键断裂和立体定向质子化反应是协调一致的,并且有效地,只有一种稳定的中间体,即 C3-gemdiolate 复合物。我们解释酶动力学结果的主要结论是,gemdiolate 可能代表难以捉摸的 Michaelis-Menten 样复合物,对应于经验 Km(=Kc),通过断键转化为产物,与观察到的催化速率一致的立体定向质子化。© 2018 Wiley Periodicals, Inc.











































 京公网安备 11010802027423号
京公网安备 11010802027423号