Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Complex strain induced structural changes observed in fibrin assembled in human plasma
Nanoscale ( IF 5.8 ) Pub Date : 2018-05-14 00:00:00 , DOI: 10.1039/c8nr00353j G. Portale 1, 2, 3, 4 , J. Torbet 5, 6, 7, 8
Nanoscale ( IF 5.8 ) Pub Date : 2018-05-14 00:00:00 , DOI: 10.1039/c8nr00353j G. Portale 1, 2, 3, 4 , J. Torbet 5, 6, 7, 8
Affiliation
|
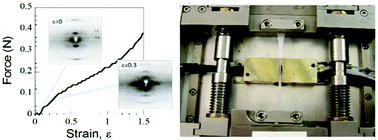
The structure of the core scaffold of blood clots, the interlinked 3-dimensional network of fibrin fibers, is modified by mechanical forces generated by platelet driven clot retraction, wound repair and shear stress through blood flow. Here X-ray diffraction is used to investigate how uniaxial strain, ε (ε = extension/original length), alters fiber structure in highly aligned human plasma clots covalently cross-linked by Factor XIIIa. Three stretch sensitive axially repeating structures are identified. Firstly, the foundation structure with an initial ≈22 nm axial repeat stretches, fades then disappears at ε ≈ 0.40. A second, lengthened transitory structure emerges at the low strains (ε ≈ 0.20) believed to be developed by cells. Finally, a third shortened structure appears after relaxation. Simultaneously as strain progresses an increasing fraction of molecules become axially disordered. Weak off-axis diffraction maxima indicate the presence of lateral ordering up to ε = 0.40 that partially recovers after relaxation. The reappearance of both axial and lateral order on relaxation demonstrates a surprising resilience in structure. In view of the range and importance of fibrin's functions, this structural heterogeneity, triggered in vivo by cell traction or shear stress, is likely to be of clinical significance.
中文翻译:

复杂应变诱导在人血浆中组装的纤维蛋白中观察到的结构变化
血凝块核心支架的结构,即纤维蛋白纤维的3维互连网络,由血小板驱动的血凝块收缩,伤口修复和通过血流产生的剪切应力产生的机械力所改变。在这里,X射线衍射用于研究单轴应变ε(ε =延伸长度/原始长度)如何改变由因子XIIIa共价交联的高度对齐的人类血浆中的纤维结构。确定了三个拉伸敏感的轴向重复结构。首先,最初的≈22纳米轴向重复伸展的基础结构,变淡然后在消失ε听,说:0.40。在低应变(ε≈0.20)被认为是由细胞形成的。最后,松弛后出现第三种缩短的结构。同时,随着应变的进行,越来越多的分子在轴向上变得无序。弱的离轴衍射最大值表明存在高达ε = 0.40的横向有序现象,松弛后部分恢复。轴向和横向次序在松弛上的重新出现证明了结构中令人惊讶的弹性。考虑到纤维蛋白功能的范围和重要性,这种由细胞牵引或剪切应力在体内触发的结构异质性可能具有临床意义。
更新日期:2018-05-14
中文翻译:

复杂应变诱导在人血浆中组装的纤维蛋白中观察到的结构变化
血凝块核心支架的结构,即纤维蛋白纤维的3维互连网络,由血小板驱动的血凝块收缩,伤口修复和通过血流产生的剪切应力产生的机械力所改变。在这里,X射线衍射用于研究单轴应变ε(ε =延伸长度/原始长度)如何改变由因子XIIIa共价交联的高度对齐的人类血浆中的纤维结构。确定了三个拉伸敏感的轴向重复结构。首先,最初的≈22纳米轴向重复伸展的基础结构,变淡然后在消失ε听,说:0.40。在低应变(ε≈0.20)被认为是由细胞形成的。最后,松弛后出现第三种缩短的结构。同时,随着应变的进行,越来越多的分子在轴向上变得无序。弱的离轴衍射最大值表明存在高达ε = 0.40的横向有序现象,松弛后部分恢复。轴向和横向次序在松弛上的重新出现证明了结构中令人惊讶的弹性。考虑到纤维蛋白功能的范围和重要性,这种由细胞牵引或剪切应力在体内触发的结构异质性可能具有临床意义。











































 京公网安备 11010802027423号
京公网安备 11010802027423号