Electrochemistry Communications ( IF 4.7 ) Pub Date : 2018-05-12 , DOI: 10.1016/j.elecom.2018.05.016 Kun Xiong , Liping Huang , Yuan Gao , Haidong Zhang , Yue Zhuo , Huizhen Shen , Yao Wang , Lishan Peng , Zidong Wei
|
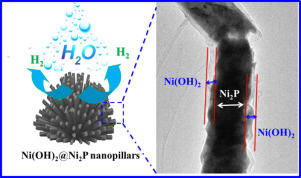
A thin-layer of Ni(OH)2 was in situ formed on the surface of Ni2P nanopillars (Ni(OH)2@Ni2P/E-NF) via a facile hydrothermal treatment in H2O. Ni(OH)2@Ni2P/E-NF exhibits superior activity for the hydrogen evolution reaction with an overpotential of only 43 mV to achieve 10 mA cm−2 and a good durability during the long-term operation in an alkaline medium. The excellent performance of Ni(OH)2@Ni2P/E-NF is likely ascribed to the strong interfacial coupling of Ni(OH)2 and Ni2P, which promotes the dissociation of water and concomitantly converts hydrogen intermediates (Had) to H2.
中文翻译:

在磷化镍纳米柱上形成氢氧化镍薄层以释放氢
的Ni(OH)的薄层2是原位形成的Ni的表面上2个P纳米柱(镍(OH)2 @Ni 2 P / E-NF)经由一个浅显的水热处理H中2 O.的Ni(OH )2 Ni 2 P / E-NF对氢析出反应显示出优异的活性,在碱性介质中的长期操作过程中,仅达到43 mV的超电势即可达到10 mA cm -2,并且具有良好的耐久性。Ni(OH)2 @Ni 2 P / E-NF的优异性能可能归因于Ni(OH)2和Ni 2的强界面耦合P促进水的离解,并同时将氢中间体(H ad)转化为H 2。











































 京公网安备 11010802027423号
京公网安备 11010802027423号