Applied Catalysis B: Environment and Energy ( IF 20.2 ) Pub Date : 2018-05-12 , DOI: 10.1016/j.apcatb.2018.05.013 Juanjuan Liu , Victor Fung , Yong Wang , Kaimin Du , Shiran Zhang , Luan Nguyen , Yu Tang , Jie Fan , De-en Jiang , Franklin Feng Tao
|
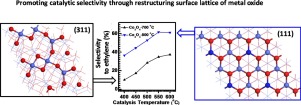
Pursuit of high catalytic selectivity is paramount in the design of catalysts for green chemical processes towards minimizing the production of undesired products. We demonstrated that catalytic selectivity for production of alkene through oxidative dehydrogenation of alkane on transition metal oxides can be promoted through tailoring the surface lattice of the oxide catalyst. Selectivity for production of ethylene through oxidative dehydrogenation (ODH) of ethane on Co3O4 nanocrystals can be substantially increased by 30%–35% via temperature-mediated reconstruction of surface lattice of Co3O4. Co3O4 nanocrystals formed at 800 °C leads to smooth, flat crystal plane with predominantly exposed (111) facet in contrast to high Miller index (311) facet of Co3O4 formed at ≤700 °C, revealed by environmental transmission electron microscopy. Isotope-labelled experiments suggest that the higher catalytic selectivity on the (111) facet results from the lower activity of its surface lattice oxygen atoms. Consistent with these experimental results, DFT calculations suggest low activity of surface lattice oxygen atoms and high activation barriers for adsorption and dissociation of CH bond on the (111) surface in contrast to (311). Upon the activation of C
H on (311), the stronger binding of ethylene on more active, under-coordinated surface lattice oxygen atoms of (311) forms a robust “deprotonated ethylene glycol”-like intermediate on (311) with a rate-limiting desorption barrier to the formation of ethylene. Compared to (311), the kinetically favorable desorption of bound ethylene species from (111) surface well rationalized the higher selectivity for production of ethylene on (111) than (311). These findings demonstrate that temperature-mediated tailoring of the surface lattice for a transition metal oxide nanocatalyst is a promising approach in pursuing high selectivity in oxidative dehydrogenation of hydrocarbons.
中文翻译:

通过重组表面晶格促进过渡金属氧化物的催化选择性
追求高催化选择性在绿色化学过程的催化剂设计中至关重要,以最大程度地减少不良产物的产生。我们证明了通过调整氧化物催化剂的表面晶格可以促进通过在过渡金属氧化物上进行烷烃的氧化脱氢来生产烯烃的催化选择性。通过温度介导的Co 3 O 4表面晶格重构,可以通过在Co 3 O 4纳米晶体上的乙烷氧化脱氢(ODH)生产乙烯的选择性可以大大提高30%–35%。钴3 O 4通过环境透射电子显微镜观察,与在≤700°C时形成的Co 3 O 4的高米勒指数(311)晶面相反,在800°C时形成的纳米晶导致光滑,平坦的晶面,且主要暴露(111)晶面。同位素标记的实验表明(111)小平面上较高的催化选择性是由于其表面晶格氧原子的活性较低所致。与这些实验结果一致,与(311)相比,DFT计算表明表面晶格中氧原子的活性较低,并且对于(111)表面上的C H键的吸附和解离具有较高的活化势垒。在激活C时
在(311)上的H上,乙烯在(311)的更活泼,配位不足的表面晶格氧原子上的较强结合会在(311)上形成坚固的“去质子化乙二醇”样中间体,并具有限速解吸屏障乙烯的形成。与(311)相比,从(111)表面动力学吸附的键合乙烯具有良好的脱附性,这使在(111)上生产乙烯的选择性比(311)高。这些发现表明,温度介导的过渡金属氧化物纳米催化剂表面晶格的剪裁是在烃类的氧化脱氢中追求高选择性的有前途的方法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号