当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Hydrogen Sulfide as a Scavenger of Sulfur Atomic Cation
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2018-05-11 00:00:00 , DOI: 10.1021/acs.jpca.8b02923 Ryan C. Fortenberry 1, 2 , Tarek Trabelsi 3 , Joseph S. Francisco 3
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2018-05-11 00:00:00 , DOI: 10.1021/acs.jpca.8b02923 Ryan C. Fortenberry 1, 2 , Tarek Trabelsi 3 , Joseph S. Francisco 3
Affiliation
|
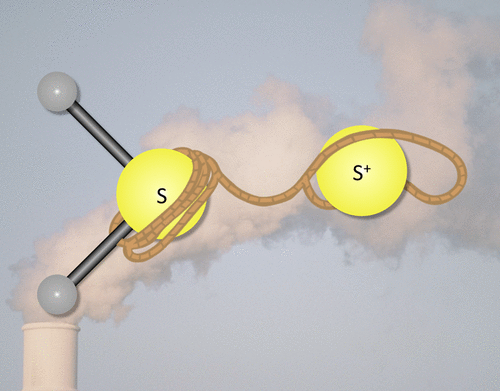
The well-studied hydrogen sulfide molecule is shown here for the first time to form a S–S bond barrierlessly with sulfur atomic cation to produce stable H2SS+, a compound for which there is nearly no literature data. Previous work has shown that the reaction of hydrogen sulfide with neutral atomic sulfur will likely only take place at high pressures. Conversely, this work shows that hydrogen sulfide will readily bind with atomic sulfur cation first through the 1 4A″ state from association of H2S with S+(4S) and then will relax to the nearly degenerate 1 2A′ or 1 2A″ states. S+(4S) + H2S lies 29.5 kcal/mol above the 1 4A″ H2SS+ minimum. The 1 4A″ H2SS+ minimum in the S–S bond is also directly intersected by the doublet potential energy surface. As the S–S bond shortens in the association, the 1 2A′ and 1 2A″ states split, falling 33.5 and 26.4 kcal/mol, respectively, below the 1 4A″ state. Hence, this work is opening the door for novel synthesis of S–S bonds or potential removal of the common H2S toxin/pollutant through concatenation and subsequent precipitation.
中文翻译:

硫化氢作为硫原子阳离子的清除剂
首次展示了经过充分研究的硫化氢分子,它与硫原子阳离子无障碍地形成S–S键,从而生成稳定的H 2 SS +,该化合物几乎没有文献数据。先前的工作表明,硫化氢与中性原子硫的反应可能只会在高压下进行。相反,这项工作表明,硫化氢将首先通过H 2 S与S +(4 S)的缔合而首先通过1 4 A''状态与原子硫阳离子结合,然后松弛到几乎简并的1 2 A'或1 2 A''状态。小号+(4 S)+ H 2 S位于最小1 4 A''H 2 SS +之上29.5 kcal / mol 。S–S键中的1 4 A''H 2 SS +最小值也直接与双峰势能面相交。如在关联的S-S键缩短,1 2 A'和1名2 A“指出分裂,分别下降33.5和26.4千卡/摩尔,低于1名4 A”的状态。因此,这项工作为新的硫键合成或通过级联和随后的沉淀潜在去除常见的H 2 S毒素/污染物打开了大门。
更新日期:2018-05-11
中文翻译:

硫化氢作为硫原子阳离子的清除剂
首次展示了经过充分研究的硫化氢分子,它与硫原子阳离子无障碍地形成S–S键,从而生成稳定的H 2 SS +,该化合物几乎没有文献数据。先前的工作表明,硫化氢与中性原子硫的反应可能只会在高压下进行。相反,这项工作表明,硫化氢将首先通过H 2 S与S +(4 S)的缔合而首先通过1 4 A''状态与原子硫阳离子结合,然后松弛到几乎简并的1 2 A'或1 2 A''状态。小号+(4 S)+ H 2 S位于最小1 4 A''H 2 SS +之上29.5 kcal / mol 。S–S键中的1 4 A''H 2 SS +最小值也直接与双峰势能面相交。如在关联的S-S键缩短,1 2 A'和1名2 A“指出分裂,分别下降33.5和26.4千卡/摩尔,低于1名4 A”的状态。因此,这项工作为新的硫键合成或通过级联和随后的沉淀潜在去除常见的H 2 S毒素/污染物打开了大门。











































 京公网安备 11010802027423号
京公网安备 11010802027423号