当前位置:
X-MOL 学术
›
ACS Med. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Profiling and Application of Photoredox C(sp3)–C(sp2) Cross-Coupling in Medicinal Chemistry
ACS Medicinal Chemistry Letters ( IF 3.5 ) Pub Date : 2018-05-07 00:00:00 , DOI: 10.1021/acsmedchemlett.8b00183 Rui Zhang 1 , Guoqing Li 1 , Michael Wismer 2 , Petr Vachal 1 , Steven L. Colletti 1 , Zhi-Cai Shi 1
ACS Medicinal Chemistry Letters ( IF 3.5 ) Pub Date : 2018-05-07 00:00:00 , DOI: 10.1021/acsmedchemlett.8b00183 Rui Zhang 1 , Guoqing Li 1 , Michael Wismer 2 , Petr Vachal 1 , Steven L. Colletti 1 , Zhi-Cai Shi 1
Affiliation
|
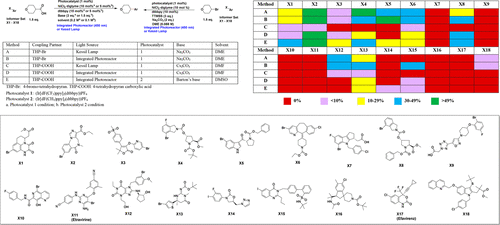
Recent visible-light photoredox catalyzed C(sp3)–C(sp2) cross-coupling provides a novel transformation to potentially enable the synthesis of medicinal chemistry targets. Here, we report a profiling study of photocatalytic C(sp3)–C(sp2) cross-coupling, both decarboxylative coupling and cross-electrophile coupling, with 18 pharmaceutically relevant aryl halides by using either Kessil lamp or our newly developed integrated photoreactor. Integrated photoreactor accelerates reaction rate and improves reaction success rate. Cross-electrophile coupling gives higher success rate with broad substrate scope on alkyl halides than that of the decarboxylative coupling. In addition, a successful application example on a discovery program demonstrates the efficient synthesis of medicinal chemistry targets via photocatalytic C(sp3)–C(sp2) cross-coupling by using our integrated photoreactor.
中文翻译:

Photoredox C(sp 3)–C(sp 2)交叉偶联在药物化学中的分析和应用
最近的可见光光氧化还原催化的C(sp 3)–C(sp 2)交叉偶联提供了一种新型的转化方法,可以潜在地实现药物化学靶标的合成。在这里,我们报告了光催化C(sp 3)–C(sp 2)通过使用Kessil灯或我们新开发的集成光反应器,与18种药学上相关的芳基卤化物进行交叉偶联(包括脱羧偶联和亲电子偶联)。集成的光反应器可加快反应速度并提高反应成功率。跨亲电偶联比脱羧偶联具有更高的成功率,在烷基卤上具有较宽的底物范围。此外,在发现程序上成功的应用实例证明了使用我们集成的光反应器通过光催化C(sp 3)–C(sp 2)交叉偶联可以有效合成药物化学目标。
更新日期:2018-05-07
中文翻译:

Photoredox C(sp 3)–C(sp 2)交叉偶联在药物化学中的分析和应用
最近的可见光光氧化还原催化的C(sp 3)–C(sp 2)交叉偶联提供了一种新型的转化方法,可以潜在地实现药物化学靶标的合成。在这里,我们报告了光催化C(sp 3)–C(sp 2)通过使用Kessil灯或我们新开发的集成光反应器,与18种药学上相关的芳基卤化物进行交叉偶联(包括脱羧偶联和亲电子偶联)。集成的光反应器可加快反应速度并提高反应成功率。跨亲电偶联比脱羧偶联具有更高的成功率,在烷基卤上具有较宽的底物范围。此外,在发现程序上成功的应用实例证明了使用我们集成的光反应器通过光催化C(sp 3)–C(sp 2)交叉偶联可以有效合成药物化学目标。











































 京公网安备 11010802027423号
京公网安备 11010802027423号