当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Efficient syntheses of alpha- and beta-C-nucleosides and the origin of anomeric selectivity†‡
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2018-05-11 00:00:00 , DOI: 10.1039/c8qo00165k Tongchao Liu 1, 2, 3, 4, 5 , Zhengdan Zhu 6, 7, 8, 9, 10 , Huanming Ren 4, 5, 8, 9, 10 , Yabin Chen 4, 5, 8, 9, 10 , Guohua Chen 4, 11, 12, 13 , Maosheng Cheng 1, 2, 3, 4 , Dongmei Zhao 1, 2, 3, 4 , Jingkang Shen 4, 5, 8, 9, 10 , Weiliang Zhu 6, 7, 8, 9, 10 , Bing Xiong 4, 5, 8, 9, 10 , Yue-Lei Chen 4, 5, 8, 9, 10
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2018-05-11 00:00:00 , DOI: 10.1039/c8qo00165k Tongchao Liu 1, 2, 3, 4, 5 , Zhengdan Zhu 6, 7, 8, 9, 10 , Huanming Ren 4, 5, 8, 9, 10 , Yabin Chen 4, 5, 8, 9, 10 , Guohua Chen 4, 11, 12, 13 , Maosheng Cheng 1, 2, 3, 4 , Dongmei Zhao 1, 2, 3, 4 , Jingkang Shen 4, 5, 8, 9, 10 , Weiliang Zhu 6, 7, 8, 9, 10 , Bing Xiong 4, 5, 8, 9, 10 , Yue-Lei Chen 4, 5, 8, 9, 10
Affiliation
|
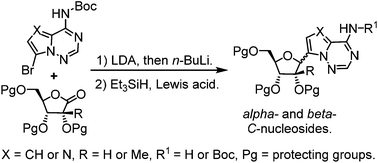
C-Nucleosides constitute a valuable class of compounds in biological and medicinal chemistry studies. We report herein a new and efficient synthesis of both alpha- and beta-C-nucleosides with high anomeric selectivity from N6-Boc protected purine analogues. The synthetic approach features a carefully designed lithiation and silane reduction sequence. The anomeric stereochemistry outcome is dictated by the protecting group of sugar lactones. Computational studies suggest that previously neglected interactions between partially positively-charged silane and the substitutions on a sugar moiety play important roles in the anomeric selectivity of silane-mediated C-nucleoside synthesis.
中文翻译:

有效合成α-和β-C-核苷以及异头选择性的起源† ‡
C-核苷在生物学和药物化学研究中构成一类有价值的化合物。我们在这里报道了一种新的有效的合成方法,它可以从N 6 -Boc保护的嘌呤类似物中合成具有高异头选择性的α-和β-C-核苷。合成方法具有精心设计的锂化和硅烷还原顺序。异头异构体立体化学结果由糖内酯的保护基决定。计算研究表明,先前被忽略的部分带正电荷的硅烷与糖部分上的取代基之间的相互作用在硅烷介导的C-核苷合成的端基异构体选择性中起重要作用。
更新日期:2018-05-11
中文翻译:

有效合成α-和β-C-核苷以及异头选择性的起源† ‡
C-核苷在生物学和药物化学研究中构成一类有价值的化合物。我们在这里报道了一种新的有效的合成方法,它可以从N 6 -Boc保护的嘌呤类似物中合成具有高异头选择性的α-和β-C-核苷。合成方法具有精心设计的锂化和硅烷还原顺序。异头异构体立体化学结果由糖内酯的保护基决定。计算研究表明,先前被忽略的部分带正电荷的硅烷与糖部分上的取代基之间的相互作用在硅烷介导的C-核苷合成的端基异构体选择性中起重要作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号