当前位置:
X-MOL 学术
›
ACS Infect. Dis.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Effects of the 1-N-(4-Amino-2S-hydroxybutyryl) and 6′-N-(2-Hydroxyethyl) Substituents on Ribosomal Selectivity, Cochleotoxicity, and Antibacterial Activity in the Sisomicin Class of Aminoglycoside Antibiotics
ACS Infectious Diseases ( IF 4.0 ) Pub Date : 2018-04-30 00:00:00 , DOI: 10.1021/acsinfecdis.8b00052 Amr Sonousi 1 , Vikram A. Sarpe 1 , Margarita Brilkova 2 , Jochen Schacht 3 , Andrea Vasella 4 , Erik C. Böttger 2 , David Crich 1
ACS Infectious Diseases ( IF 4.0 ) Pub Date : 2018-04-30 00:00:00 , DOI: 10.1021/acsinfecdis.8b00052 Amr Sonousi 1 , Vikram A. Sarpe 1 , Margarita Brilkova 2 , Jochen Schacht 3 , Andrea Vasella 4 , Erik C. Böttger 2 , David Crich 1
Affiliation
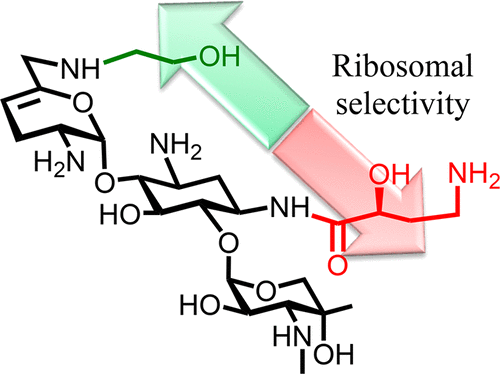
|
Syntheses of the 6′-N-(2-hydroxyethyl) and 1-N-(4-amino-2S-hydroxybutyryl) derivatives of the 4,6-aminoglycoside sisomicin and that of the doubly modified 1-N-(4-amino-2S-hydroxybutyryl)-6′-N-(2-hydroxyethyl) derivative known as plazomicin are reported together with their antibacterial and antiribosomal activities and selectivities. The 6′-N-(2-hydroxyethyl) modification results in a moderate increase in prokaryotic/eukaryotic ribosomal selectivity, whereas the 1-N-(4-amino-2S-hydroxybutyryl) modification has the opposite effect. When combined in plazomicin, the effects of the two groups on ribosomal selectivity cancel each other out, leading to the prediction that plazomicin will exhibit ototoxicity comparable to those of the parent and the current clinical aminoglycoside antibiotics gentamicin and tobramycin, as borne out by ex vivo studies with mouse cochlear explants. The 6′-N-(2-hydroxyethyl) modification restores antibacterial activity in the presence of the AAC(6′) aminoglycoside-modifying enzymes, while the 1-N-(4-amino-2S-hydroxybutyryl) modification overcomes resistance to the AAC(2′) class but is still affected to some extent by the AAC(3) class. Neither modification is able to circumvent the ArmA ribosomal methyltransferase-induced aminoglycoside resistance. The use of phenyltriazenyl protection for the secondary amino group of sisomicin facilitates the synthesis of each derivative and their characterization through the provision of sharp NMR spectra for all intermediates.
中文翻译:

效果1- Ñ - (4-氨基-2-小号-羟基丁酰基)和6'- ñ - (2-羟乙基)在氨基糖苷类抗生素的西索米星类上核糖体选择性,Cochleotoxicity,与抗菌活性的取代基
4,6-氨基糖苷西索霉素和双修饰的1- N-(4-的6' - N-(2-羟乙基)和1- N-(4-氨基-2 S-羟基丁酰基)衍生物的合成报道了称为吡唑米星的氨基-2 S-羟基丁酰基)-6'- N-(2-羟基乙基)衍生物以及它们的抗菌和抗核糖体活性和选择性。6'- N-(2-羟乙基)修饰导致原核/真核生物核糖体选择性的适度提高,而1 - N-(4-氨基-2 S-羟基丁酰基)修饰具有相反的作用。当与吡唑米星合用时,两组对核糖体选择性的影响相互抵消,从而导致预测,吡唑米星将表现出与亲本和目前临床氨基糖苷类抗生素庆大霉素和妥布霉素相当的耳毒性,这在离体研究中得到了证实。小鼠耳蜗外植体的研究。在存在AAC(6')氨基糖苷修饰酶的情况下,6'- N-(2-羟乙基)修饰可恢复抗菌活性,而在1 - N-(4-amino-2 S(羟基丁酰基)修饰克服了对AAC(2')类的抗性,但仍在某种程度上受AAC(3)类的影响。两种修饰都不能规避ArmA核糖体甲基转移酶诱导的氨基糖苷耐药性。通过为所有中间体提供清晰的NMR光谱,对sisomicin的仲氨基使用苯基三氮烯基保护基可促进每种衍生物的合成及其表征。
更新日期:2018-04-30
中文翻译:

效果1- Ñ - (4-氨基-2-小号-羟基丁酰基)和6'- ñ - (2-羟乙基)在氨基糖苷类抗生素的西索米星类上核糖体选择性,Cochleotoxicity,与抗菌活性的取代基
4,6-氨基糖苷西索霉素和双修饰的1- N-(4-的6' - N-(2-羟乙基)和1- N-(4-氨基-2 S-羟基丁酰基)衍生物的合成报道了称为吡唑米星的氨基-2 S-羟基丁酰基)-6'- N-(2-羟基乙基)衍生物以及它们的抗菌和抗核糖体活性和选择性。6'- N-(2-羟乙基)修饰导致原核/真核生物核糖体选择性的适度提高,而1 - N-(4-氨基-2 S-羟基丁酰基)修饰具有相反的作用。当与吡唑米星合用时,两组对核糖体选择性的影响相互抵消,从而导致预测,吡唑米星将表现出与亲本和目前临床氨基糖苷类抗生素庆大霉素和妥布霉素相当的耳毒性,这在离体研究中得到了证实。小鼠耳蜗外植体的研究。在存在AAC(6')氨基糖苷修饰酶的情况下,6'- N-(2-羟乙基)修饰可恢复抗菌活性,而在1 - N-(4-amino-2 S(羟基丁酰基)修饰克服了对AAC(2')类的抗性,但仍在某种程度上受AAC(3)类的影响。两种修饰都不能规避ArmA核糖体甲基转移酶诱导的氨基糖苷耐药性。通过为所有中间体提供清晰的NMR光谱,对sisomicin的仲氨基使用苯基三氮烯基保护基可促进每种衍生物的合成及其表征。











































 京公网安备 11010802027423号
京公网安备 11010802027423号