European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2018-05-10 , DOI: 10.1016/j.ejmech.2018.05.010 Qinhuai Lai , Yuxi Wang , Ruixue Wang , Weirong Lai , Liangze Tang , Yiran Tao , Yu Liu , Ruirui Zhang , Luyi Huang , Haotian Xiang , Shaoxue Zeng , Lantu Gou , Hao Chen , Yuqin Yao , Jinliang Yang
|
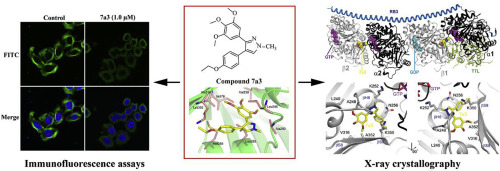
Tubulin inhibitors that target the colchicine binding site continue to emerge as promising anticancer agents. In this study, based on the anti-proliferative activities, a novel tubulin inhibitor 7a3 targeting the colchicine binding site was designed, synthesized, and optimized from a series of novel cis-restricted pyrazole analogues of combretastatin A-4. The structure-activity relationships (SARs) of these newly synthesized compounds are summarized indicating that the methyl substituent at the N1 position and deamination were significantly important for the anti-proliferative efficacy. The optimized compound 7a3 exhibited the ability to arrest the cell cycle in the G2/M phase, induce cell apoptosis, and inhibit cell migration in tumour cells. The results of the immunofluorescence analysis using confocal microscopy and the tubulin polymerization assay revealed that tubulin assembly was disrupted by 7a3 in vitro. Furthermore, the targeting identification of 7a3 was illuminated by solving the crystal structure of 7a3 in complex with tubulin at a resolution of 3.2 Å (PDB code 5Z4U), which confirmed the result of molecular docking and further demonstrated that 7a3 binds to the site of colchicine. Moreover, the pharmacokinetic analysis in mouse plasma showed that 7a3 rapidly reached a peak concentration at 0.25 h after intraperitoneal administration, and the T1/2, Cmax, and AUC0-inf were 1.67 ± 0.28 h, 882 ± 71 ng mL-1, and 1166 ± 129 h ng·mL-1, respectively, after a single-dose administration analysed by liquid chromatography-tandem mass spectrometry (LC/MS/MS). In addition, the in vivo study indicated that 7a3 significantly inhibited the tumour growth of the SK-OV-3 xenograft in a nude mouse model. In conclusion, our study proved 7a3 to be a potential microtubule-targeting drug for cancer therapy. The SARs and mechanism of action studies of 7a3 based on the X-ray co-crystal structure provided insights into the next-generation tubulin inhibitors for cancer therapy.
中文翻译:

针对秋水仙碱结合位点的新型微管蛋白抑制剂7a3的设计,合成和生物学评估
靶向秋水仙碱结合位点的微管蛋白抑制剂继续作为有希望的抗癌药出现。在这项研究中,基于抗增殖活性,从康布雷他汀A-4的一系列新型顺式限制性吡唑类似物设计,合成和优化了针对秋水仙碱结合位点的新型微管蛋白抑制剂7a3。总结了这些新合成化合物的结构活性关系(SAR),表明N1位置的甲基取代基和脱氨基作用对于抗增殖功效非常重要。优化的化合物7a3具有在G2 / M期阻止细胞周期,诱导细胞凋亡和抑制肿瘤细胞中细胞迁移的能力。使用共聚焦显微镜和微管蛋白聚合测定的免疫荧光分析结果表明,体外7a3破坏了微管蛋白装配。此外,靶向识别7A3通过求解的晶体结构照亮7A3在3.2埃的分辨率(PDB代码5Z4U),这证实分子对接的结果,并进一步表明,与微管蛋白在复杂7A3结合于秋水仙碱的部位。此外,小鼠血浆中的药代动力学分析表明7a3腹腔内给药后,在0.25小时迅速达到峰值浓度,和T 1/2,C最大值,AUC和0-INF是1.67±0.28小时,882±71纳克毫升-1和1166±129伍·毫升-通过液相色谱-串联质谱法(LC / MS / MS)分析单次给药后分别如图1所示。另外,体内研究表明7a3在裸鼠模型中显着抑制了SK-OV-3异种移植物的肿瘤生长。总之,我们的研究证明7a3是潜在的靶向微管的癌症治疗药物。7a3的SAR和作用机理研究 基于X射线共晶体的结构为了解下一代用于癌症治疗的微管蛋白抑制剂提供了见识。









































 京公网安备 11010802027423号
京公网安备 11010802027423号