当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Discovery of 4,7-Diamino-5-(4-phenoxyphenyl)-6-methylene-pyrimido[5,4-b]pyrrolizines as Novel Bruton’s Tyrosine Kinase Inhibitors
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2018-05-01 00:00:00 , DOI: 10.1021/acs.jmedchem.8b00441 Yu Xue 1 , Peiran Song 2 , Zilan Song , Aoli Wang 3 , Linjiang Tong , Meiyu Geng 2, 4 , Jian Ding 2, 4 , Qingsong Liu 3 , Liping Sun 1 , Hua Xie 4 , Ao Zhang 2, 4
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2018-05-01 00:00:00 , DOI: 10.1021/acs.jmedchem.8b00441 Yu Xue 1 , Peiran Song 2 , Zilan Song , Aoli Wang 3 , Linjiang Tong , Meiyu Geng 2, 4 , Jian Ding 2, 4 , Qingsong Liu 3 , Liping Sun 1 , Hua Xie 4 , Ao Zhang 2, 4
Affiliation
|
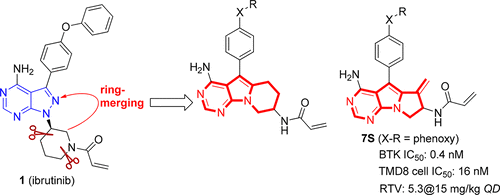
An alternative medicinal chemistry approach was conducted on Bruton’s tyrosine kinase (BTK) inhibitor 1 (ibrutinib) by merging the pyrazolo[3,4-d]pyrimidine component into a tricyclic skeleton. Two types of compounds were prepared, and their biochemical activities on BTK as well as stereochemistry effects were determined. Structural optimization focusing on the reactive binding group to BTK Cys481 and on the metabolic site guided by metabolic study were conducted. 7S was identified as the most potent showing an IC50 value of 0.4 nM against BTK and 16 nM against BTK-dependent TMD8 cells. Compared to 1, 7S was slightly more selective with strong inhibition on the B-cell receptor signaling pathway. In a TMD8 cell-derived animal xenograft model, 7S showed a relative tumor volume of 5.3 at 15 mg/kg QD dosage that was more efficacious than 1 (RTV 6.6) at a higher dose of 25 mg/kg QD. All these results suggest 7S as a new BTK inhibitor worthy of further profiling.
中文翻译:

发现4,7-二氨基-5-(4-苯氧基苯基)-6-亚甲基-嘧啶[5,4- b ]吡咯烷酮作为新型布鲁顿酪氨酸激酶抑制剂
通过将吡唑并[3,4- d ]嘧啶组分合并成三环骨架,对布鲁顿酪氨酸激酶(BTK)抑制剂1(依鲁替尼)进行了另一种药物化学处理。制备了两种类型的化合物,并确定了它们对BTK的生化活性以及立体化学作用。进行了结构优化,重点是与BTK Cys481的反应性结合基团和以代谢研究为指导的代谢位点。7S被认为是最有效的,对BTK的IC 50值为0.4 nM,对BTK依赖的TMD8细胞的IC 50为16 nM。相比1,7S对B细胞受体信号通路的抑制作用强一些。在源自TMD8细胞的动物异种移植模型中,7S在15 mg / kg QD剂量下显示出5.3的相对肿瘤体积,在25 mg / kg QD较高剂量下比1(RTV 6.6)更有效。所有这些结果表明7S是一种值得进一步分析的新型BTK抑制剂。
更新日期:2018-05-01
中文翻译:

发现4,7-二氨基-5-(4-苯氧基苯基)-6-亚甲基-嘧啶[5,4- b ]吡咯烷酮作为新型布鲁顿酪氨酸激酶抑制剂
通过将吡唑并[3,4- d ]嘧啶组分合并成三环骨架,对布鲁顿酪氨酸激酶(BTK)抑制剂1(依鲁替尼)进行了另一种药物化学处理。制备了两种类型的化合物,并确定了它们对BTK的生化活性以及立体化学作用。进行了结构优化,重点是与BTK Cys481的反应性结合基团和以代谢研究为指导的代谢位点。7S被认为是最有效的,对BTK的IC 50值为0.4 nM,对BTK依赖的TMD8细胞的IC 50为16 nM。相比1,7S对B细胞受体信号通路的抑制作用强一些。在源自TMD8细胞的动物异种移植模型中,7S在15 mg / kg QD剂量下显示出5.3的相对肿瘤体积,在25 mg / kg QD较高剂量下比1(RTV 6.6)更有效。所有这些结果表明7S是一种值得进一步分析的新型BTK抑制剂。











































 京公网安备 11010802027423号
京公网安备 11010802027423号