Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2018-05-09 , DOI: 10.1016/j.bioorg.2018.04.027 Subhash Chander , Cheng-Run Tang , Ashok Penta , Ping Wang , Deepak P. Bhagwat , Nicolas Vanthuyne , Muriel Albalat , Payal Patel , Sanskruti Sankpal , Yong-Tang Zheng , Murugesan Sankaranarayanan
|
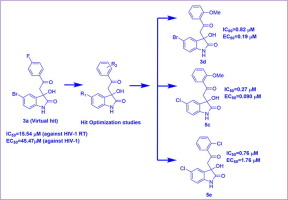
In the current study, twenty-two compounds based upon 3-hydroxy-3-(2-oxo-2-phenylethyl)indolin-2-one nucleus were designed, synthesized and in vitro evaluated for HIV-1 RT inhibition and anti-HIV-1 activity. Compounds 3d, 5c and 5e demonstrated encouraging potency against RT enzyme as well as HIV-1 in low micromolar to nanomolar concentration with good to excellent safety index. Structure activity relationship studies revealed that halogens such as bromo or chloro at 5th the position of oxindole ring remarkably enhanced the potency against RT. Moreover, methoxy or chloro groups at the ortho position of phenyl ring also significantly favored RT inhibition activity. Seven compounds (3b, 3c, 3d, 3e, 5b, 5c and 5e) with better anti-HIV-1 potency were tested against the mutant HIV-1K103N strain. The putative binding mode, as well as interaction patterns of the best active compound 5c with wild HIV-1 RT were studied via docking studies.
中文翻译:

3-羟基吲哚-2-酮类似物作为潜在抗HIV-1药物的命中优化研究
在本研究中,设计,合成了22种基于3-羟基-3-(2-氧代-2-苯基乙基)吲哚-2-酮核的化合物,并在体外评估了其对HIV-1 RT的抑制作用和抗HIV的作用。 -1活动。化合物3d,5c和5e在低微摩尔至纳摩尔浓度下显示出令人鼓舞的针对RT酶和HIV-1的效力,并具有良好至极佳的安全指数。结构活性关系研究表明,在羟吲哚环第5位的卤素(如溴或氯)显着提高了对RT的效力。此外,在苯环邻位的甲氧基或氯基团也显着有利于RT抑制活性。七种化合物(3b,3c,3d,3e,5b,5c和5e)对突变的HIV-1 K103N菌株测试了具有更好抗HIV-1效力的药物。通过对接研究对推定的结合模式以及最佳活性化合物5c与野生HIV-1 RT的相互作用模式进行了研究。











































 京公网安备 11010802027423号
京公网安备 11010802027423号