当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Organocatalytic asymmetric [3 + 2] annulation of 1,4-dithiane-2,5-diol with azlactones: access to chiral dihydrothiophen-2(3H)-one derivatives†
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2018-05-09 00:00:00 , DOI: 10.1039/c8qo00305j Ji-cong Yu 1, 2, 3, 4 , Le-mao Yu 1, 2, 3, 4 , Xiao-yun Zhao 1, 2, 3, 4 , Lu Gan 1, 2, 3, 4 , Wei-wei Zhu 1, 2, 3, 4 , Ze-chen Wang 1, 3, 4, 5 , Rui Wang 4, 6, 7, 8, 9 , Xianxing Jiang 1, 2, 3, 4
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2018-05-09 00:00:00 , DOI: 10.1039/c8qo00305j Ji-cong Yu 1, 2, 3, 4 , Le-mao Yu 1, 2, 3, 4 , Xiao-yun Zhao 1, 2, 3, 4 , Lu Gan 1, 2, 3, 4 , Wei-wei Zhu 1, 2, 3, 4 , Ze-chen Wang 1, 3, 4, 5 , Rui Wang 4, 6, 7, 8, 9 , Xianxing Jiang 1, 2, 3, 4
Affiliation
|
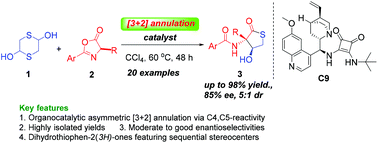
Herein, the first organocatalyst-mediated enantioselective [3 + 2] annulations of 1,4-dithiane-2,5-diol with various azlactones utilizing C4 and C5 reactivity using squaramide as the catalyst in a ring-opening manner have been reported. This protocol provides convenient access to enantioenriched substituted dihydrothiophen-2(3H)-one derivatives. Excellent isolated yields and moderate to good enantioselectivities have been achieved.
中文翻译:

1,4-二硫代-2,5-二醇与a内酯的有机催化不对称[3 + 2]环化反应:获得手性二氢噻吩-2(3 H)-一衍生物†
在本文中,已经报道了第一批有机催化剂介导的1,4-二噻吩-2,5-二醇与各种氮杂内酯的对映体选择[3 + 2]环化,该反应使用方酰胺以开环方式利用C4和C5反应性进行C4和C5反应性。该协议为对映体富集的取代的二氢噻吩-2(3 H)-one衍生物提供了方便的访问途径。已获得优异的分离产率和中等至良好的对映选择性。
更新日期:2018-05-09
中文翻译:

1,4-二硫代-2,5-二醇与a内酯的有机催化不对称[3 + 2]环化反应:获得手性二氢噻吩-2(3 H)-一衍生物†
在本文中,已经报道了第一批有机催化剂介导的1,4-二噻吩-2,5-二醇与各种氮杂内酯的对映体选择[3 + 2]环化,该反应使用方酰胺以开环方式利用C4和C5反应性进行C4和C5反应性。该协议为对映体富集的取代的二氢噻吩-2(3 H)-one衍生物提供了方便的访问途径。已获得优异的分离产率和中等至良好的对映选择性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号