当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Design of Conformationally Constrained Acyl Sulfonamide Isosteres: Identification of N-([1,2,4]Triazolo[4,3-a]pyridin-3-yl)methane-sulfonamides as Potent and Selective hNaV1.7 Inhibitors for the Treatment of Pain
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2018-05-08 00:00:00 , DOI: 10.1021/acs.jmedchem.7b01826 Thilo Focken 1 , Sultan Chowdhury 1 , Alla Zenova 1 , Michael E. Grimwood 1 , Christine Chabot 2 , Tao Sheng 1 , Ivan Hemeon 1 , Shannon M. Decker 1 , Michael Wilson 1 , Paul Bichler 1 , Qi Jia 1 , Shaoyi Sun 1 , Clint Young 1 , Sophia Lin 1 , Samuel J. Goodchild 1 , Noah G. Shuart 1 , Elaine Chang 1 , Zhiwei Xie 1 , Bowen Li 1 , Kuldip Khakh 1 , Girish Bankar 1 , Matthew Waldbrook 1 , Rainbow Kwan 1 , Karen Nelkenbrecher 1 , Parisa Karimi Tari 1 , Navjot Chahal 1 , Luis Sojo 1 , C. Lee Robinette 1 , Andrew D. White 3 , Chien-An Chen 3 , Yi Zhang 3 , Jodie Pang 2 , Jae H. Chang 2 , David H. Hackos 2 , J. P. Johnson 1 , Charles J. Cohen 1 , Daniel F. Ortwine 2 , Daniel P. Sutherlin 2 , Christoph M. Dehnhardt 1 , Brian S. Safina 2
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2018-05-08 00:00:00 , DOI: 10.1021/acs.jmedchem.7b01826 Thilo Focken 1 , Sultan Chowdhury 1 , Alla Zenova 1 , Michael E. Grimwood 1 , Christine Chabot 2 , Tao Sheng 1 , Ivan Hemeon 1 , Shannon M. Decker 1 , Michael Wilson 1 , Paul Bichler 1 , Qi Jia 1 , Shaoyi Sun 1 , Clint Young 1 , Sophia Lin 1 , Samuel J. Goodchild 1 , Noah G. Shuart 1 , Elaine Chang 1 , Zhiwei Xie 1 , Bowen Li 1 , Kuldip Khakh 1 , Girish Bankar 1 , Matthew Waldbrook 1 , Rainbow Kwan 1 , Karen Nelkenbrecher 1 , Parisa Karimi Tari 1 , Navjot Chahal 1 , Luis Sojo 1 , C. Lee Robinette 1 , Andrew D. White 3 , Chien-An Chen 3 , Yi Zhang 3 , Jodie Pang 2 , Jae H. Chang 2 , David H. Hackos 2 , J. P. Johnson 1 , Charles J. Cohen 1 , Daniel F. Ortwine 2 , Daniel P. Sutherlin 2 , Christoph M. Dehnhardt 1 , Brian S. Safina 2
Affiliation
|
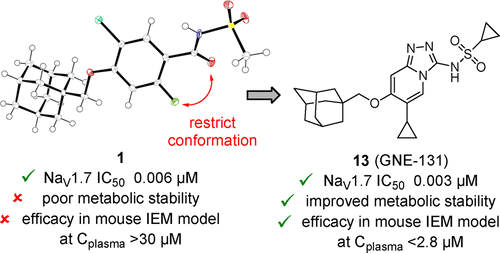
The sodium channel NaV1.7 has emerged as a promising target for the treatment of pain based on strong genetic validation of its role in nociception. In recent years, a number of aryl and acyl sulfonamides have been reported as potent inhibitors of NaV1.7, with high selectivity over the cardiac isoform NaV1.5. Herein, we report on the discovery of a novel series of N-([1,2,4]triazolo[4,3-a]pyridin-3-yl)methanesulfonamides as selective NaV1.7 inhibitors. Starting with the crystal structure of an acyl sulfonamide, we rationalized that cyclization to form a fused heterocycle would improve physicochemical properties, in particular lipophilicity. Our design strategy focused on optimization of potency for block of NaV1.7 and human metabolic stability. Lead compounds 10, 13 (GNE-131), and 25 showed excellent potency, good in vitro metabolic stability, and low in vivo clearance in mouse, rat, and dog. Compound 13 also displayed excellent efficacy in a transgenic mouse model of induced pain.
中文翻译:

构象约束的酰基磺酰胺类异构体的设计:N -([1,2,4]三唑并[4,3 - a ]吡啶-3-基)甲烷-磺酰胺类化合物作为强效和选择性h Na V 1.7抑制剂的治疗鉴定疼痛
钠通道Na V 1.7已经成为治疗疼痛的有前途的靶点,这是基于其在伤害感受中作用的强大遗传学验证。近年来,已经报道了许多芳基和酰基磺酰胺作为Na V 1.7的有效抑制剂,与心脏同工型Na V 1.5相比具有很高的选择性。在此,我们报道了一系列新的N -([1,2,4]三唑并[4,3 - a ]吡啶-3-基)甲磺酰胺作为选择性Na V的发现。1.7抑制剂。从酰基磺酰胺的晶体结构开始,我们合理地认为,形成稠合杂环的环化将改善物理化学性质,特别是亲脂性。我们的设计策略集中于优化Na V 1.7的效价和人体代谢稳定性。铅化合物10,13(GNE-131),和25显示出优异的效力,良好的体外代谢稳定性,和低体内在小鼠,大鼠,和狗的间隙。化合物13在诱导性疼痛的转基因小鼠模型中也显示出优异的功效。
更新日期:2018-05-08
中文翻译:

构象约束的酰基磺酰胺类异构体的设计:N -([1,2,4]三唑并[4,3 - a ]吡啶-3-基)甲烷-磺酰胺类化合物作为强效和选择性h Na V 1.7抑制剂的治疗鉴定疼痛
钠通道Na V 1.7已经成为治疗疼痛的有前途的靶点,这是基于其在伤害感受中作用的强大遗传学验证。近年来,已经报道了许多芳基和酰基磺酰胺作为Na V 1.7的有效抑制剂,与心脏同工型Na V 1.5相比具有很高的选择性。在此,我们报道了一系列新的N -([1,2,4]三唑并[4,3 - a ]吡啶-3-基)甲磺酰胺作为选择性Na V的发现。1.7抑制剂。从酰基磺酰胺的晶体结构开始,我们合理地认为,形成稠合杂环的环化将改善物理化学性质,特别是亲脂性。我们的设计策略集中于优化Na V 1.7的效价和人体代谢稳定性。铅化合物10,13(GNE-131),和25显示出优异的效力,良好的体外代谢稳定性,和低体内在小鼠,大鼠,和狗的间隙。化合物13在诱导性疼痛的转基因小鼠模型中也显示出优异的功效。









































 京公网安备 11010802027423号
京公网安备 11010802027423号