Journal of the American Society for Mass Spectrometry ( IF 3.1 ) Pub Date : 2018-05-08 , DOI: 10.1007/s13361-018-1973-3 Maha T. Abutokaikah 1 , Joseph W. Frye 1 , John Tschampel 2 , Jordan M. Rabus 1 , Benjamin J. Bythell 1
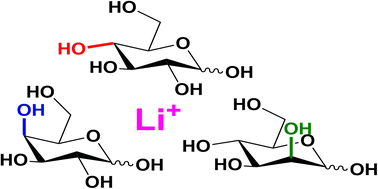
We characterize the primary fragmentation reactions of three isomeric lithiated D-hexose sugars (glucose, galactose, and mannose) utilizing tandem mass spectrometry, regiospecific labeling, and theory. We provide evidence that these three isomers populate similar fragmentation pathways to produce the abundant cross-ring cleavage peaks (0,2A1 and 0,3A1). These pathways are highly consistent with the prior literature (Hofmeister et al. J. Am. Chem. Soc. 113, 5964–5970, 1991, Bythell et al. J. Am. Soc. Mass Spectrom. 28, 688–703, 2017, Rabus et al. Phys. Chem. Chem. Phys. 19, 25643–25652, 2017) and the present labeling data. However, the structure-specific energetics and rate-determining steps of these reactions differ as a function of precursor sugar and anomeric configuration. The lowest energy water loss pathways involve loss of the anomeric oxygen to furnish B1 ions. For glucose and galactose, the lithiated α-anomers generate ketone structures at C2 in a concerted reaction involving a 1,2-migration of the C2-H to the anomeric carbon (C1). In contrast, the β-anomers are predicted to form 1,3-anhydroglucose/galactose B1 ion structures. Initiation of the water loss reactions from each anomeric configuration requires distinct reactive conformers, resulting in different product ion structures. Inversion of the stereochemistry at C2 has marked consequences. Both lithiated mannose forms expel water to form 1,2-anhydromannose B1 ions with the newly formed epoxide group above the ring. Additionally, provided water loss is not instantaneous, the α-anomer can also isomerize to generate a ketone structure at C2 in a concerted reaction involving a 1,2-migration of the C2-H to C1. This product is indistinguishable to that from α-glucose. The energetics and interplay of these pathways are discussed.
ᅟ
中文翻译:

锂化己糖单糖的片段化途径
我们利用串联质谱,区域特异性标记和理论表征了三种异构化的锂化D-己糖(葡萄糖,半乳糖和甘露糖)的主要裂解反应。我们提供的证据表明,这三个异构体组成相似的片段化途径,以产生丰富的交叉环裂解峰(0.2 A 1和0.3 A 1)。这些途径是与现有文献高度一致(霍夫迈斯特等,J。化学会志。113,5964-5970,1991年,Bythell等人J. PM。SOC。质谱Spectrom。28,688-703,2017年,Rabus等人的PHY。化学式化学物理。19,25643-25652,2017年)和当前的标签数据。然而,这些反应的结构特异性能量学和速率确定步骤根据前体糖和异头构型的不同而不同。最低的能量失水途径涉及损失异头氧以提供B 1离子。对于葡萄糖和半乳糖,锂化的α-端基异构体在协同反应中在C2处生成酮结构,该协同反应涉及C2-H向异头碳(C1)的1,2-迁移。相反,预测β-端基异构体形成1,3-脱水葡萄糖/半乳糖B 1。离子结构。从每个端基异构体构型开始失水反应需要不同的反应性构象异构体,从而导致不同的产物离子结构。在C2处立体化学的倒转具有明显的后果。两种锂化的甘露糖均会排出水,形成1,2-脱水甘露糖B 1离子,并在环上方形成新形成的环氧基。另外,如果水分损失不是瞬时的,则α-端基异构体还可以在涉及C2-H向C1的1,2-迁移的协同反应中异构化以在C2处生成酮结构。该产物与α-葡萄糖没有区别。讨论了这些途径的能量学和相互作用。

ᅟ











































 京公网安备 11010802027423号
京公网安备 11010802027423号