当前位置:
X-MOL 学术
›
Biomacromolecules
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Arginine-Containing Surfactant-Like Peptides: Interaction with Lipid Membranes and Antimicrobial Activity
Biomacromolecules ( IF 5.5 ) Pub Date : 2018-05-08 00:00:00 , DOI: 10.1021/acs.biomac.8b00391 Valeria Castelletto 1 , Ruth H. Barnes 1 , Kimon-Andreas Karatzas 1 , Charlotte J. C. Edwards-Gayle 1 , Francesca Greco 1 , Ian W. Hamley 1 , Robert Rambo 2 , Jani Seitsonen 3 , Janne Ruokolainen 3
Biomacromolecules ( IF 5.5 ) Pub Date : 2018-05-08 00:00:00 , DOI: 10.1021/acs.biomac.8b00391 Valeria Castelletto 1 , Ruth H. Barnes 1 , Kimon-Andreas Karatzas 1 , Charlotte J. C. Edwards-Gayle 1 , Francesca Greco 1 , Ian W. Hamley 1 , Robert Rambo 2 , Jani Seitsonen 3 , Janne Ruokolainen 3
Affiliation
|
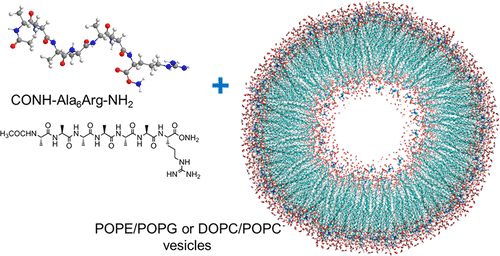
The activity of antimicrobial peptides stems from their interaction with bacterial membranes, which are disrupted according to a number of proposed mechanisms. Here, we investigate the interaction of a model antimicrobial peptide that contains a single arginine residue with vesicles containing model lipid membranes. The surfactant-like peptide Ala6-Arg (A6R) is studied in the form where both termini are capped (CONH-A6R-NH2, capA6R) or uncapped (NH2-A6R-OH, A6R). Lipid membranes are selected to correspond to model anionic membranes (POPE/POPG) resembling those in bacteria or model zwitterionic membranes (POPC/DOPC) similar to those found in mammalian cells. Viable antimicrobial agents should show activity against anionic membranes but not zwitterionic membranes. We find, using small-angle X-ray scattering (SAXS) and cryogenic-TEM (transmission electron microscopy) that, uniquely, capA6R causes structuring of anionic membranes due to the incorporation of the peptide in the lipid bilayer with peptide β-sheet conformation revealed by circular dichroism spectroscopy (CD). There is a preferential interaction of the peptide with POPG (which is the only anionic lipid in the systems studied) due to electrostatic interactions and bidentate hydrogen bonding between arginine guanidinium and lipid phosphate groups. At a certain composition, this peptide leads to the remarkable tubulation of zwitterionic phosphatidylcholine (PC) vesicles, which is ascribed to the interaction of the peptide with the outer lipid membrane, which occurs without penetration into the membrane. In contrast, peptide A6R has a minimal influence on the anionic lipid membranes (and no β-sheet peptide structure is observed) but causes thinning (lamellar decorrelation) of zwitterionic membranes. We also investigated the cytotoxicity (to fibroblasts) and antimicrobial activity of these two peptides against model Gram positive and Gram negative bacteria. A strong selective antimicrobial activity against Gram positive Listeria monocytogenes, which is an important food-borne pathogen, is observed for capA6R. Peptide A6R is active against all three studied bacteria. The activity of the peptides against bacteria and mammalian cells is related to the specific interactions uncovered through our SAXS, cryo-TEM, and CD measurements. Our results highlight the exquisite sensitivity to the charge distribution in these designed peptides and its effect on the interaction with lipid membranes bearing different charges, and ultimately on antimicrobial activity.
中文翻译:

含精氨酸的表面活性剂样肽:与脂质膜的相互作用和抗菌活性
抗菌肽的活性源于它们与细菌膜的相互作用,而细菌膜根据许多提出的机制而被破坏。在这里,我们调查了包含单个精氨酸残基的模型抗菌肽与包含模型脂质膜的囊泡的相互作用。研究了表面活性剂样肽Ala 6 -Arg(A 6 R),其中两个末端均被封端(CONH-A 6 R-NH 2,capA 6 R)或未封端(NH 2 -A 6 R-OH, A 6R)。选择脂质膜以对应于类似于细菌中的阴离子膜的模型阴离子膜(POPE / POPG)或类似于哺乳动物细胞中的膜的两性离子膜(POPC / DOPC)。可行的抗菌剂应显示出对阴离子膜的活性,但对两性离子膜没有活性。我们发现,使用小角度X射线散射(SAXS)和低温TEM(透射电子显微镜),capA 6是唯一的。R导致阴离子膜结构化,这是由于脂双分子层中的肽掺入了具有由圆二色光谱(CD)揭示的肽β-折叠构象的肽。由于精氨酸胍盐和脂质磷酸基团之间的静电相互作用和双齿氢键,该肽与POPG(这是研究的系统中唯一的阴离子脂质)存在优先相互作用。在一定的组成下,该肽导致两性离子磷脂酰胆碱(PC)囊泡的显着小管形成,这归因于该肽与外部脂质膜的相互作用,这种相互作用没有渗透到膜中。相反,肽A 6R对阴离子脂质膜的影响极小(并且未观察到β-sheet肽结构),但会导致两性离子膜变薄(薄片去相关)。我们还研究了这两种肽对革兰氏阳性和革兰氏阴性细菌的细胞毒性(对成纤维细胞的影响)和抗菌活性。对于capA 6 R ,观察到了对革兰氏阳性单核细胞增生李斯特菌(一种重要的食源性病原体)的强选择性抗菌活性。肽A 6R对所有三种研究的细菌均具有活性。肽对细菌和哺乳动物细胞的活性与通过我们的SAXS,cryo-TEM和CD测量揭示的特定相互作用有关。我们的结果强调了对这些设计肽段中电荷分布的极佳敏感性及其对与带有不同电荷的脂质膜相互作用的影响,并最终对抗菌活性产生了影响。
更新日期:2018-05-08
中文翻译:

含精氨酸的表面活性剂样肽:与脂质膜的相互作用和抗菌活性
抗菌肽的活性源于它们与细菌膜的相互作用,而细菌膜根据许多提出的机制而被破坏。在这里,我们调查了包含单个精氨酸残基的模型抗菌肽与包含模型脂质膜的囊泡的相互作用。研究了表面活性剂样肽Ala 6 -Arg(A 6 R),其中两个末端均被封端(CONH-A 6 R-NH 2,capA 6 R)或未封端(NH 2 -A 6 R-OH, A 6R)。选择脂质膜以对应于类似于细菌中的阴离子膜的模型阴离子膜(POPE / POPG)或类似于哺乳动物细胞中的膜的两性离子膜(POPC / DOPC)。可行的抗菌剂应显示出对阴离子膜的活性,但对两性离子膜没有活性。我们发现,使用小角度X射线散射(SAXS)和低温TEM(透射电子显微镜),capA 6是唯一的。R导致阴离子膜结构化,这是由于脂双分子层中的肽掺入了具有由圆二色光谱(CD)揭示的肽β-折叠构象的肽。由于精氨酸胍盐和脂质磷酸基团之间的静电相互作用和双齿氢键,该肽与POPG(这是研究的系统中唯一的阴离子脂质)存在优先相互作用。在一定的组成下,该肽导致两性离子磷脂酰胆碱(PC)囊泡的显着小管形成,这归因于该肽与外部脂质膜的相互作用,这种相互作用没有渗透到膜中。相反,肽A 6R对阴离子脂质膜的影响极小(并且未观察到β-sheet肽结构),但会导致两性离子膜变薄(薄片去相关)。我们还研究了这两种肽对革兰氏阳性和革兰氏阴性细菌的细胞毒性(对成纤维细胞的影响)和抗菌活性。对于capA 6 R ,观察到了对革兰氏阳性单核细胞增生李斯特菌(一种重要的食源性病原体)的强选择性抗菌活性。肽A 6R对所有三种研究的细菌均具有活性。肽对细菌和哺乳动物细胞的活性与通过我们的SAXS,cryo-TEM和CD测量揭示的特定相互作用有关。我们的结果强调了对这些设计肽段中电荷分布的极佳敏感性及其对与带有不同电荷的脂质膜相互作用的影响,并最终对抗菌活性产生了影响。









































 京公网安备 11010802027423号
京公网安备 11010802027423号