Tetrahedron ( IF 2.1 ) Pub Date : 2018-05-08 , DOI: 10.1016/j.tet.2018.04.031 Paul R. Rablen
|
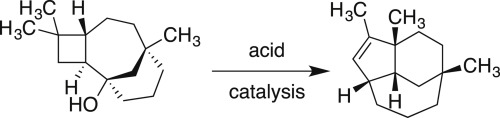
Acid-catalyzed conversion of caryolan-1-ol to isoclovene involves a multi-step carbocation rearrangement. Electronic structure calculations show that the pathway proceeds through an initial 3° carbocation, as well as a series of three other 3° carbocations. The key stage in which the ring structure is rearranged occurs not as might initially be imagined, in two separate steps with the intermediacy of a 2° carbocation, but rather in a single, concerted but highly asynchronous dyotropic rearrangement. The transition structure for this dyotropic rearrangement strongly resembles the 2° carbocation that would be involved in a stepwise mechanism. However, the dyotropic rearrangement is stereochemically unusual. While one of the bond migrations is suprafacial, as expected, the other is effectively antarafacial. This unusual stereochemical outcome is enforced by the geometric constraints of the polycyclic structure.
中文翻译:

酸催化的caryolan-1-ol转化为异丁烯酮:多步碳正离子重排的计算研究
酸催化的Caryolan-1-ol转化为异丁烯酮涉及多步碳正离子重排。电子结构计算表明,该途径通过了最初的3°碳正离子,以及一系列其他三个3°碳正离子。环结构重排的关键阶段并不像最初想象的那样发生,而是在两个相互独立的步骤中,中间发生2°碳正离子化,而是发生在一个单一的,协调的但高度异步的致密重排中。该致伸缩重排的过渡结构非常类似于逐步机制中涉及的2°碳正离子化。然而,发散重排在立体化学上是不寻常的。正如预期的那样,尽管其中一种键迁移是超分子的,但另一种实际上是反亲的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号