当前位置:
X-MOL 学术
›
ACS Earth Space Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Technetium Stabilization in Low-Solubility Sulfide Phases: A Review
ACS Earth and Space Chemistry ( IF 3.4 ) Pub Date : 2018-05-08 00:00:00 , DOI: 10.1021/acsearthspacechem.8b00015 Carolyn I. Pearce 1 , Jonathan P. Icenhower 2 , R. Matthew Asmussen 1 , Paul G. Tratnyek 3 , Kevin M. Rosso 1 , Wayne W. Lukens 4 , Nikolla P. Qafoku 1
ACS Earth and Space Chemistry ( IF 3.4 ) Pub Date : 2018-05-08 00:00:00 , DOI: 10.1021/acsearthspacechem.8b00015 Carolyn I. Pearce 1 , Jonathan P. Icenhower 2 , R. Matthew Asmussen 1 , Paul G. Tratnyek 3 , Kevin M. Rosso 1 , Wayne W. Lukens 4 , Nikolla P. Qafoku 1
Affiliation
|
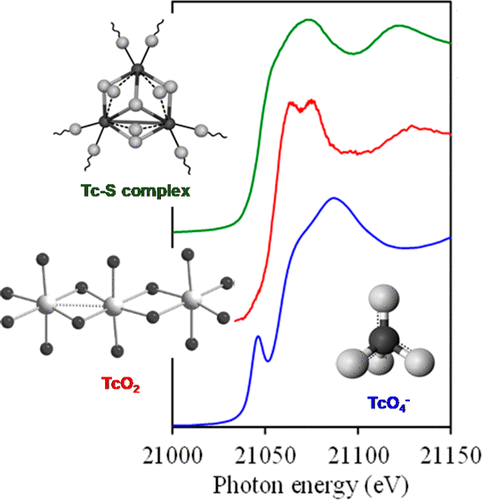
Technetium contamination remains a major environmental problem at nuclear reprocessing sites, e.g., the Hanford Site, Washington, USA. At these sites, Tc is present in liquid waste destined for immobilization in a waste form or has been released into the subsurface environment. The high environmental risk associated with Tc is due to its long half-life (214 000 years) and the mobility of the oxidized anionic species Tc(VII)O4–. Under reducing conditions, TcO4– is readily reduced to Tc(IV), which commonly exists as a relatively insoluble and therefore immobile, hydrous Tc-oxide (TcO2·nH2O). The stability of Tc(IV) sequestered as solid phases depends on the solubility of the solid and susceptibility to reoxidation to TcO4–, which in turn depend on the (biogeo)chemical conditions of the environment and/or nuclear waste streams. Unfortunately, the solubility of crystalline TcO2 or amorphous TcO2·H2O is still above the maximum contaminant level (MCL) established by the U.S. EPA (900 pCi/L), and the kinetics of TcO2 oxidative dissolution can be on the order of days to years. In addition to oxygen, sulfur can form complexes that significantly affect the adsorption, solubility, and reoxidation potential of Tc, especially Tc(IV). The principal technetium sulfides are TcS2 and Tc2S7, but much less is known about the mechanisms of formation, stabilization, and reoxidation of Tc-sulfides. A common assumption is that sulfides are less soluble than their oxyhydrous counterparts. Determination of the molecular structure of Tc2S7 in particular has been hampered by the propensity of this phase to precipitate as an amorphous substance. Recent work indicates that the oxidation state of Tc in Tc2S7 is Tc(IV), in apparent contradiction to its nominal stoichiometry. Technetium is relatively immobile in reduced sediments and soils, but in many cases the exact sink for Tc has not been identified. Experiments and modeling have demonstrated that both abiotic and biologic mechanisms can exert strong controls on Tc mobility and that Tc binding or uptake into sulfide phases can occur. These and similar investigations also show that extended exposure to oxidizing conditions results in transformation of sulfide-stabilized Tc(IV) to a Tc(IV)O2-like phase without formation of measurable dissolved TcO4–, suggesting a solid-state transformation in which Tc(IV)-associated sulfide is preferentially oxidized before the Tc(IV) cation. This transformation of Tc(IV)-sulfides to Tc(IV)-oxides may be the main process that limits remobilization of Tc as Tc(VII)O4–. The efficacy of the final waste form to retain Tc also strongly depends on the ability of oxidizing species to enter the waste and convert Tc(IV) to Tc(VII). Many waste form designs are reducing (e.g., cementitious waste forms such as salt stone) and, therefore, attempt to restrict access of oxidizing species such that diffusion is the rate-limiting step in remobilization of Tc.
中文翻译:

低溶解度硫化物相中的稳定性:综述
nuclear污染仍然是核后处理厂(例如美国华盛顿州汉福德厂)的主要环境问题。在这些场所,Tc以废料的形式存在于固定化的废液中,或已释放到地下环境中。与Tc相关的高环境风险归因于其长的半衰期(214 000年)和氧化的阴离子物质Tc(VII)O 4 –的迁移性。在还原条件下,TcO 4 –容易还原为Tc(IV),通常以相对不溶的,因此不可移动的含水Tc-氧化物(TcO 2 · n H 2)的形式存在。O)。固相形式的Tc(IV)的稳定性取决于固体的溶解度和对再氧化为TcO 4 –的敏感性,而这又取决于环境和/或核废料流的(生物地理)化学条件。不幸的是,结晶态TcO 2或非晶态TcO 2 ·H 2 O的溶解度仍高于美国EPA(900 pCi / L)确定的最大污染物水平(MCL),而TcO 2氧化溶解的动力学可能取决于数天至数年。除氧外,硫还可能形成显着影响Tc(尤其是Tc(IV))的吸附,溶解度和再氧化潜能的络合物。主要的硫化tech是TcS 2Tc 2 S 7和Tc 2 S 7,但对Tc硫化物的形成,稳定和再氧化的机理知之甚少。一个普遍的假设是,硫化物的溶解度低于其含氧的对应物。Tc 2 S 7的分子结构的确定尤其受到该相作为无定形物质沉淀的倾向的阻碍。最近的工作表明Tc在2 S 7中的Tc的氧化态Tc(IV)与它的名义化学计量存在明显矛盾。reduced在减少的沉积物和土壤中相对不动,但在许多情况下,仍未确定Tc的确切汇。实验和建模表明,非生物和生物机制均可对Tc的流动性施加强有力的控制,并且Tc结合或被硫化物相吸收。这些研究和类似研究还表明,长时间暴露于氧化条件会导致硫化物稳定的Tc(IV)转变为类似Tc(IV)O 2的相,而不会形成可测量的溶解TcO 4 –,表明存在固态转变,其中与Tc(IV)相关的硫化物在Tc(IV)阳离子之前被优先氧化。锝的这种转化(IV)-sulfides到TC(IV)-oxides可以是主进程,锝的限制再活化在Tc(VII)O 4 - 。最终废物形式保留Tc的功效在很大程度上还取决于氧化性物质进入废物并将Tc(IV)转化为Tc(VII)的能力。许多废物形式的设计正在减少(例如,水泥废物形式,例如盐石),因此试图限制氧化性物质的进入,从而扩散是Tc固定化的限速步骤。
更新日期:2018-05-08
中文翻译:

低溶解度硫化物相中的稳定性:综述
nuclear污染仍然是核后处理厂(例如美国华盛顿州汉福德厂)的主要环境问题。在这些场所,Tc以废料的形式存在于固定化的废液中,或已释放到地下环境中。与Tc相关的高环境风险归因于其长的半衰期(214 000年)和氧化的阴离子物质Tc(VII)O 4 –的迁移性。在还原条件下,TcO 4 –容易还原为Tc(IV),通常以相对不溶的,因此不可移动的含水Tc-氧化物(TcO 2 · n H 2)的形式存在。O)。固相形式的Tc(IV)的稳定性取决于固体的溶解度和对再氧化为TcO 4 –的敏感性,而这又取决于环境和/或核废料流的(生物地理)化学条件。不幸的是,结晶态TcO 2或非晶态TcO 2 ·H 2 O的溶解度仍高于美国EPA(900 pCi / L)确定的最大污染物水平(MCL),而TcO 2氧化溶解的动力学可能取决于数天至数年。除氧外,硫还可能形成显着影响Tc(尤其是Tc(IV))的吸附,溶解度和再氧化潜能的络合物。主要的硫化tech是TcS 2Tc 2 S 7和Tc 2 S 7,但对Tc硫化物的形成,稳定和再氧化的机理知之甚少。一个普遍的假设是,硫化物的溶解度低于其含氧的对应物。Tc 2 S 7的分子结构的确定尤其受到该相作为无定形物质沉淀的倾向的阻碍。最近的工作表明Tc在2 S 7中的Tc的氧化态Tc(IV)与它的名义化学计量存在明显矛盾。reduced在减少的沉积物和土壤中相对不动,但在许多情况下,仍未确定Tc的确切汇。实验和建模表明,非生物和生物机制均可对Tc的流动性施加强有力的控制,并且Tc结合或被硫化物相吸收。这些研究和类似研究还表明,长时间暴露于氧化条件会导致硫化物稳定的Tc(IV)转变为类似Tc(IV)O 2的相,而不会形成可测量的溶解TcO 4 –,表明存在固态转变,其中与Tc(IV)相关的硫化物在Tc(IV)阳离子之前被优先氧化。锝的这种转化(IV)-sulfides到TC(IV)-oxides可以是主进程,锝的限制再活化在Tc(VII)O 4 - 。最终废物形式保留Tc的功效在很大程度上还取决于氧化性物质进入废物并将Tc(IV)转化为Tc(VII)的能力。许多废物形式的设计正在减少(例如,水泥废物形式,例如盐石),因此试图限制氧化性物质的进入,从而扩散是Tc固定化的限速步骤。



























 京公网安备 11010802027423号
京公网安备 11010802027423号