Molecular Catalysis ( IF 3.9 ) Pub Date : 2018-05-08 , DOI: 10.1016/j.mcat.2018.02.019 C.A. Ferretti , J.I. Di Cosimo
|
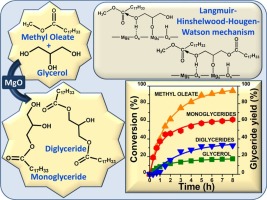
The reaction kinetics of the liquid-phase synthesis of glyceryl monooleates (monoglycerides, MG) and dioleates (diglycerides, DG) from methyl oleate (a fatty acid methyl ester, FAME) and glycerol (Gly) was studied on MgO. The reaction occurs in consecutive steps, with the MG formation by glycerolysis of FAME being the first step, followed by the transesterification of MG with FAME toward DG. The reaction system was investigated at different temperatures and reactant ratios. MG yields of up to 62% were obtained at 503 K using a Gly/FAME molar ratio of 4.5. A molecular modeling based on DFT calculations was used to study the Gly and FAME adsorptions and the reaction mechanism toward MG on a MgO (100) surface. Results showed that the adsorption of hydrophilic Gly on MgO is much stronger than that of hydrophobic FAME. Two transitions states and one tetrahedral intermediate participate in the reaction pathway toward MG. Based on the DFT results, a five-step heterogeneous Langmuir-Hinshelwood-Hougen-Watson mechanism and kinetic model were postulated considering that the reaction occurs between an adsorbed Gly molecule and a FAME molecule in the liquid phase. After preliminary discrimination based on initial rates, the surface reaction steps toward MG and DG were taken as rate-limiting. The model predicts the consecutive formation of DG after glycerolysis of FAME toward MG. In addition, the model anticipates that the MG/DG ratio will increase with temperature in agreement with the catalytic results and that the only relevant adsorption enthalpy is that of Gly. The kinetic parameters derived from regression of the experimental data at different temperatures satisfactorily describe also the catalytic results obtained at different Gly/FAME ratios.
中文翻译:

油酸甲酯与甘油在MgO上转化反应的动力学和理论研究
在MgO上研究了由油酸甲酯(脂肪酸甲酯,FAME)和甘油(Gly)液相合成甘油单油酸酯(单甘油酯,MG)和二油酸酯(二甘油酯,DG)的反应动力学。该反应在连续的步骤中进行,第一步是通过FAME的甘油分解形成MG,然后是MG与FAME向DG进行酯交换。在不同的温度和反应物比例下研究了反应体系。使用4.5的Gly / FAME摩尔比,在503 K下获得高达62%的MG产率。基于DFT计算的分子模型用于研究Gly和FAME吸附以及在MgO(100)表面上对MG的反应机理。结果表明,亲水性Gly在MgO上的吸附要强于疏水性FAME。两个过渡态和一个四面体中间体参与了向MG的反应途径。根据DFT结果,考虑到液相中吸附的Gly分子和FAME分子之间发生了反应,提出了五步异质Langmuir-Hinshelwood-Hougen-Watson机理和动力学模型。在基于初始速率的初步判别后,将朝向MG和DG的表面反应步骤视为速率限制。该模型预测了FAME对MG的甘油分解后DG的连续形成。此外,该模型预计MG / DG比值将随温度的增加而增加,并与催化结果相符,并且唯一相关的吸附焓是Gly。











































 京公网安备 11010802027423号
京公网安备 11010802027423号