Analytical and Bioanalytical Chemistry ( IF 4.3 ) Pub Date : 2018-05-08 , DOI: 10.1007/s00216-018-1085-8 Urairat Koesukwiwat , Lukas Vaclavik , Katerina Mastovska
According to the European Commission directive 2006/141/EC, haloxyfop residue levels should not exceed 0.003 mg/kg in ready-to-feed infant formula, and the residue definition includes sum of haloxyfop, its esters, salts, and conjugates expressed as haloxyfop. A simple method for total haloxyfop analysis in infant formula and related ingredient matrices was developed and validated using liquid chromatography-tandem mass spectrometry (LC-MS/MS). The sample preparation consisted of an alkaline hydrolysis with methanolic sodium hydroxide to release haloxyfop (parent acid) from its bound forms prior to the extraction with acetonitrile. A mixture of magnesium sulfate (MgSO4) and sodium chloride (NaCl) (4:1, w/w) was added to the extract to induce phase separation and force the analyte into the upper acetonitrile-methanol layer and then a 1-mL aliquot was subsequently cleaned up by dispersive solid phase extraction with 150 mg of MgSO4 and 50 mg of octadecyl (C18) sorbent. The analytical procedure was developed and carefully optimized to enable low-level, total haloxyfop analysis in a variety of challenging matrices, including infant formulas and their important high-carbohydrate, high-protein, high-fat, and emulsifier ingredients. The final method was validated in two different laboratories by fortifying samples with haloxyfop and haloxyfop-methyl, which was used as a model compound simulating bound forms of the analyte. Mean recoveries of haloxyfop across all fortification levels and evaluated matrices ranged between 92.2 and 114% with repeatability, within-lab reproducibility, and reproducibility RSDs ≤ 14%. Based on the validation results, this method was capable to convert the haloxyfop ester into the parent acid in a wide range of sample types and to reliably identify and quantify total haloxyfop at the target 0.003 mg/kg level in infant formulas (both powdered and ready-to-feed liquid forms).
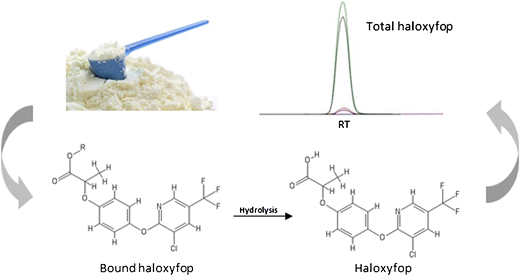
LC-MS/MS-based workflow for the determination of the total haloxyfop in infant formula and related ingredients
中文翻译:

液相色谱-串联质谱法开发婴儿配方奶粉和相关成分中的总卤氧基苯酚的方法开发和验证
根据欧盟委员会指令2006/141 / EC的规定,即食婴儿配方食品中的haloxyfop残留量不应超过0.003 mg / kg,且残留量的定义包括haloxyfop,其酯,盐和以haloxyfop表示的缀合物的总和。 。开发了一种简单的婴儿配方奶粉和相关成分矩阵中的总卤氧基苯酚分析方法,并使用液相色谱-串联质谱(LC-MS / MS)进行了验证。样品制备方法是用甲醇氢氧化钠进行碱水解,以从其结合形式释放出卤氧基f(母体酸),然后再用乙腈萃取。硫酸镁(MgSO 4)和氯化钠(NaCl)(4:1,w / w)加入萃取物中以诱导相分离,并迫使分析物进入乙腈-甲醇的上层,然后用150 mg MgSO 4和50 mg十八烷基(C 18岁)吸附剂。开发了分析程序,并对其进行了仔细的优化,以实现在各种挑战性基质中的低含量,总卤代甲氧基苯酚分析,包括婴儿配方食品及其重要的高碳水化合物,高蛋白,高脂肪和乳化剂成分。最终方法在两个不同的实验室中得到了验证,方法是分别用氟吡咯烷酮和甲基氟吡咯烷酮强化样品,而氟吡咯烷酮和甲基吡咯烷酮被用作模拟化合物的结合形式的模型化合物。在所有设防水平和评估的矩阵中,卤虫草的平均回收率在92.2%至114%之间,可重复性,实验室内可重复性和可重复性RSD≤14%。根据验证结果,

基于LC-MS / MS的工作流程,用于确定婴儿配方食品和相关成分中的总氟烷



























 京公网安备 11010802027423号
京公网安备 11010802027423号