当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Aromatic-Based Design of Highly Active and Noncalcemic Vitamin D Receptor Agonists
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2018-05-07 00:00:00 , DOI: 10.1021/acs.jmedchem.8b00337 Pranjal Gogoi 1 , Samuel Seoane 2 , Rita Sigüeiro 1, 3 , Thierry Guiberteau 4 , Miguel A. Maestro 5 , Román Pérez-Fernández 2 , Natacha Rochel 3 , Antonio Mouriño 1
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2018-05-07 00:00:00 , DOI: 10.1021/acs.jmedchem.8b00337 Pranjal Gogoi 1 , Samuel Seoane 2 , Rita Sigüeiro 1, 3 , Thierry Guiberteau 4 , Miguel A. Maestro 5 , Román Pérez-Fernández 2 , Natacha Rochel 3 , Antonio Mouriño 1
Affiliation
|
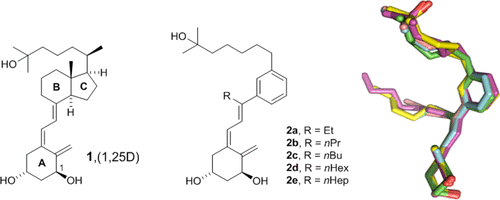
We report the design, synthesis, biological evaluation, and structural analysis of a new class of vitamin D analogues that possess an aromatic m-phenylene D-ring and an alkyl chain replacing the C-ring. A key feature of the synthetic strategy is a stereoselective Pd-catalyzed construction of the triene system in aqueous medium that allows the rapid preparation of small amounts of VDR ligands for biological screening. Analogues with the shorter (2a) and longer (2d, 2e) side chains attached to the triene system have no calcemic activity. Compound 2a binds to VDR with the same order of magnitude than calcipotriol and oxacalcitriol. It also reduces proliferation in normal and tumor cells similarly to the natural hormone 1α,25-dihydroxyvitamin D3, calcipotriol, and oxacalcitriol, suggesting preclinical studies related to hyperproliferative disorders such as psoriasis and cancer.
中文翻译:

高活性和非钙化维生素D受体激动剂的基于芳香族的设计
我们报告的设计,合成,生物学评价,和一类新的具有该芳族维生素d类似物的结构分析米亚苯基d型圈和的烷基链取代Ç形圈。合成策略的关键特征是在水介质中三烯系统的立体选择性Pd催化结构,该结构允许快速制备少量VDR配体用于生物筛选。与较短的(2a)和较长的(2d,2e)侧链连接到三烯系统的类似物没有降钙活性。化合物2a以与卡泊三醇和草酰三醇相同的数量级结合到VDR。与天然激素1α,25-二羟基维生素D 3,卡泊三醇和草酰三醇类似,它也减少正常细胞和肿瘤细胞的增殖,提示与银屑病和癌症等过度增殖性疾病有关的临床前研究。
更新日期:2018-05-07
中文翻译:

高活性和非钙化维生素D受体激动剂的基于芳香族的设计
我们报告的设计,合成,生物学评价,和一类新的具有该芳族维生素d类似物的结构分析米亚苯基d型圈和的烷基链取代Ç形圈。合成策略的关键特征是在水介质中三烯系统的立体选择性Pd催化结构,该结构允许快速制备少量VDR配体用于生物筛选。与较短的(2a)和较长的(2d,2e)侧链连接到三烯系统的类似物没有降钙活性。化合物2a以与卡泊三醇和草酰三醇相同的数量级结合到VDR。与天然激素1α,25-二羟基维生素D 3,卡泊三醇和草酰三醇类似,它也减少正常细胞和肿瘤细胞的增殖,提示与银屑病和癌症等过度增殖性疾病有关的临床前研究。











































 京公网安备 11010802027423号
京公网安备 11010802027423号