当前位置:
X-MOL 学术
›
ACS Earth Space Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Interaction of Hydrocarbons with Clays under Reservoir Conditions: In Situ Infrared and Nuclear Magnetic Resonance Spectroscopy and X-ray Diffraction for Expandable Clays with Variably Wet Supercritical Methane
ACS Earth and Space Chemistry ( IF 2.9 ) Pub Date : 2018-05-07 00:00:00 , DOI: 10.1021/acsearthspacechem.8b00039 Geoffrey M. Bowers 1 , John S. Loring , H. Todd Schaef , Eric D. Walter , Sarah D. Burton , David W. Hoyt , Sydney S. Cunniff 1 , Narasimhan Loganathan , R. James Kirkpatrick
ACS Earth and Space Chemistry ( IF 2.9 ) Pub Date : 2018-05-07 00:00:00 , DOI: 10.1021/acsearthspacechem.8b00039 Geoffrey M. Bowers 1 , John S. Loring , H. Todd Schaef , Eric D. Walter , Sarah D. Burton , David W. Hoyt , Sydney S. Cunniff 1 , Narasimhan Loganathan , R. James Kirkpatrick
Affiliation
|
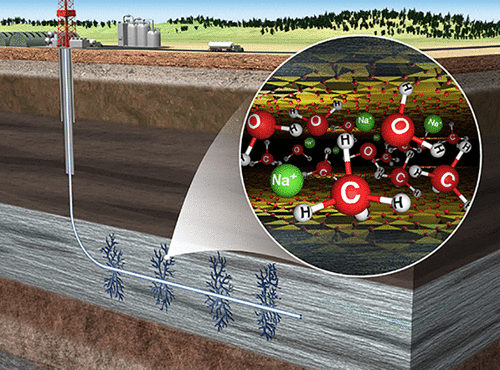
The results from novel in situ high-pressure nuclear magnetic resonance (NMR) spectroscopy, infrared (IR) spectroscopy, and X-ray diffraction (XRD) investigation of the interaction of the smectite hectorite with variably wet supercritical methane (scCH4) at 90 bar and 323 K (hydrostatic conditions equivalent to ∼1 km depth) show that CH4 occurs in the clay interlayers, in pores external to the individual clay particles, and as bulk fluid. The occupancy of each environment depends on the relative humidity (RH) of the CH4-rich fluid and the hydration energy and size of the charge-balancing cation. As RH increases, the fraction of interlayer and interparticle CH4 decreases, although with Cs+, addition of a small amount of H2O initially increases CH4 uptake. Maximum interlayer CH4 adsorption occurs when the mean basal spacing just permits methane intercalation (∼11.5 Å) and never below this basal spacing. It is also higher with divalent cations than with monovalent cations. The data show that CH4 adsorption occurs predominantly via a weak dispersion interaction with the clay and that its intercalation occurs via a passive space-filling hydrophobic mechanism. The results suggest that, under reservoir conditions, smectite interlayers may provide a reservoir for CH4 under low-water conditions.
中文翻译:

储层条件下碳氢化合物与黏土的相互作用:可变湿超临界甲烷膨胀黏土的原位红外和核磁共振谱及X射线衍射
新颖的原位高压核磁共振(NMR)光谱,红外(IR)光谱和X射线衍射(XRD)研究蒙脱石锂蒙脱石与90℃下可变湿超临界甲烷(scCH 4)相互作用的结果bar和323 K(相当于约1 km深度的静水压条件)表明,CH 4出现在粘土夹层中,单个粘土颗粒外部的孔隙中以及作为散装流体。每种环境的占有率取决于富含CH 4的流体的相对湿度(RH)以及水合能和电荷平衡阳离子的大小。随着RH的增加,层间和粒子间CH 4的分数降低,尽管Cs +,添加少量的H 2 O最初会增加CH 4的吸收。当平均基础间距仅允许甲烷嵌入(〜11.5Å)且从未低于此基础间距时,就会发生最大的层间CH 4吸附。与二价阳离子相比,二价阳离子也更高。数据表明,CH 4吸附主要通过与粘土的弱分散相互作用而发生,并且其插层通过被动的空间填充疏水机理发生。结果表明,在储层条件下,蒙脱石夹层可能为低水条件下的CH 4提供了一个储层。
更新日期:2018-05-07
中文翻译:

储层条件下碳氢化合物与黏土的相互作用:可变湿超临界甲烷膨胀黏土的原位红外和核磁共振谱及X射线衍射
新颖的原位高压核磁共振(NMR)光谱,红外(IR)光谱和X射线衍射(XRD)研究蒙脱石锂蒙脱石与90℃下可变湿超临界甲烷(scCH 4)相互作用的结果bar和323 K(相当于约1 km深度的静水压条件)表明,CH 4出现在粘土夹层中,单个粘土颗粒外部的孔隙中以及作为散装流体。每种环境的占有率取决于富含CH 4的流体的相对湿度(RH)以及水合能和电荷平衡阳离子的大小。随着RH的增加,层间和粒子间CH 4的分数降低,尽管Cs +,添加少量的H 2 O最初会增加CH 4的吸收。当平均基础间距仅允许甲烷嵌入(〜11.5Å)且从未低于此基础间距时,就会发生最大的层间CH 4吸附。与二价阳离子相比,二价阳离子也更高。数据表明,CH 4吸附主要通过与粘土的弱分散相互作用而发生,并且其插层通过被动的空间填充疏水机理发生。结果表明,在储层条件下,蒙脱石夹层可能为低水条件下的CH 4提供了一个储层。











































 京公网安备 11010802027423号
京公网安备 11010802027423号