当前位置:
X-MOL 学术
›
J. Chem. Inf. Model.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Binding of Cytotoxic Aβ25–35 Peptide to the Dimyristoylphosphatidylcholine Lipid Bilayer
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2018-05-04 00:00:00 , DOI: 10.1021/acs.jcim.8b00045 Amy K. Smith 1 , Dmitri K. Klimov 1
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2018-05-04 00:00:00 , DOI: 10.1021/acs.jcim.8b00045 Amy K. Smith 1 , Dmitri K. Klimov 1
Affiliation
|
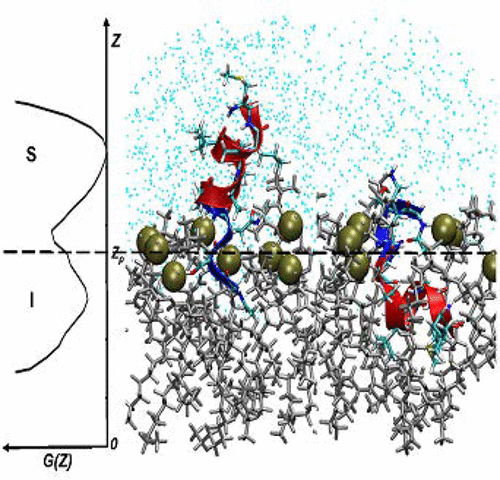
Aβ25–35 is a short, cytotoxic, and naturally occurring fragment of the Alzheimer’s Aβ peptide. To map the molecular mechanism of Aβ25–35 binding to the zwitterionic dimyristoylphosphatidylcholine (DMPC) bilayer, we have performed replica exchange with solute tempering molecular dynamics simulations using all-atom explicit membrane and water models. Consequences of sequence truncation on the binding mechanism have been measured by utilizing as a control our previous simulations probing binding of the longer peptide Aβ10–40 to the same bilayer. The most intriguing feature of Aβ25–35 binding to the DMPC bilayer is a coexistence of two bound states with strikingly different characteristics: a dominant surface-bound state and a less stable inserted state. In the surface-bound state, the peptide samples extended conformations, in which its unbound C-terminal is pointed away from the bilayer. In contrast, in the inserted state, the C-terminal resides deep in the bilayer hydrophobic core. In both states, the N-terminal remains anchored to the bilayer. Free energy landscape analysis reveals that the two states are separated by a moderate barrier, suggesting that Aβ25–35 monomer may frequently interconvert between them. The net effect of Aβ25–35 binding is a minor impact on the bilayer structure, which contrasts with the considerable bilayer perturbations induced by a longer Aβ10–40 peptide penetrating deep into the bilayer core. Therefore, we conclude that the binding mechanisms of Aβ25–35 and Aβ10–40 peptides are different. Potential implications of our results for Aβ25–35 cytotoxicity are discussed. A comparison of experimental data with our findings reveals a good agreement.
中文翻译:

细胞毒性Aβ25–35肽与二肉豆蔻酰磷脂酰胆碱脂双层的结合
Aβ25–35是阿尔茨海默氏症Aβ肽的一种短的,具有细胞毒性的天然存在的片段。为了绘制Aβ25–35结合两性离子二甲基丙二酰磷脂酰胆碱(DMPC)双层的分子机制,我们使用全原子显式膜和水模型进行了溶质回火分子动力学模拟的副本交换。已经通过利用我们先前的模拟作为对照来测量序列截短对结合机制的影响,该模拟探讨了更长的肽Aβ10-40与同一双层的结合。Aβ25-35与DMPC双层结合的最吸引人的特征是两种具有显着不同特征的结合状态共存:显性表面结合状态和不稳定的插入状态。在表面结合状态下,肽样品扩展了构象,其中未结合的C末端指向双层之外。相反,在插入状态下,C-末端位于双层疏水核的深处。在两种状态下,N端均保持锚固在双层上。自由能态分析表明,这两个状态被适度的障碍隔开,这表明Aβ25–35单体可能经常在它们之间相互转换。Aβ25–35结合的净作用对双层结构的影响很小,这与较长的Aβ10–40肽深入双层核心所引起的相当大的双层扰动相反。因此,我们得出结论,Aβ25–35和Aβ10–40肽的结合机制是不同的。讨论了我们的结果对Aβ25-35细胞毒性的潜在影响。
更新日期:2018-05-04
中文翻译:

细胞毒性Aβ25–35肽与二肉豆蔻酰磷脂酰胆碱脂双层的结合
Aβ25–35是阿尔茨海默氏症Aβ肽的一种短的,具有细胞毒性的天然存在的片段。为了绘制Aβ25–35结合两性离子二甲基丙二酰磷脂酰胆碱(DMPC)双层的分子机制,我们使用全原子显式膜和水模型进行了溶质回火分子动力学模拟的副本交换。已经通过利用我们先前的模拟作为对照来测量序列截短对结合机制的影响,该模拟探讨了更长的肽Aβ10-40与同一双层的结合。Aβ25-35与DMPC双层结合的最吸引人的特征是两种具有显着不同特征的结合状态共存:显性表面结合状态和不稳定的插入状态。在表面结合状态下,肽样品扩展了构象,其中未结合的C末端指向双层之外。相反,在插入状态下,C-末端位于双层疏水核的深处。在两种状态下,N端均保持锚固在双层上。自由能态分析表明,这两个状态被适度的障碍隔开,这表明Aβ25–35单体可能经常在它们之间相互转换。Aβ25–35结合的净作用对双层结构的影响很小,这与较长的Aβ10–40肽深入双层核心所引起的相当大的双层扰动相反。因此,我们得出结论,Aβ25–35和Aβ10–40肽的结合机制是不同的。讨论了我们的结果对Aβ25-35细胞毒性的潜在影响。











































 京公网安备 11010802027423号
京公网安备 11010802027423号