当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Intercalation and Conversion Reactions of Nanosized β-MnO2 Cathode in the Secondary Zn/MnO2 Alkaline Battery
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2018-05-03 00:00:00 , DOI: 10.1021/acs.jpcc.7b11685 Joon Kyo Seo , JaeWook Shin , Hyeseung Chung , Po Yu Meng , Xuefeng Wang , Y. Shirley Meng
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2018-05-03 00:00:00 , DOI: 10.1021/acs.jpcc.7b11685 Joon Kyo Seo , JaeWook Shin , Hyeseung Chung , Po Yu Meng , Xuefeng Wang , Y. Shirley Meng
|
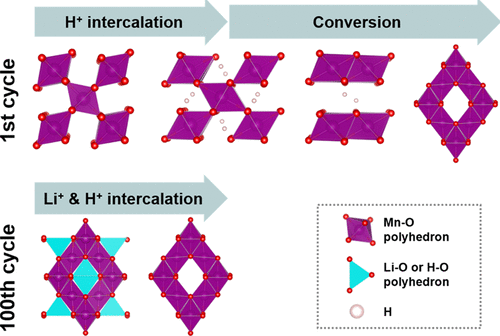
This work reports rechargeable Zn/β-MnO2 alkaline batteries as promising stationary energy storage. Unlike commercial alkaline batteries with poor cyclic performance, the nanosized β-MnO2 cathode in the mixture of LiOH and KOH electrolyte enables rechargeable reactions with high capacity. To unveil the underlying reaction mechanisms of nanosized β-MnO2, we combine thermodynamic frameworks with experimental characterization, including electrochemistry, X-ray diffraction, and X-ray photoelectron spectroscopy. The results demonstrate a series of proton intercalation reaction (β-MnO2 → γ-MnOOH) and two-phase conversion reactions (γ-MnOOH → Mn(OH)2 → λ-MnO2) during the first cycle and Li and H cointercalation in the host structure of λ-MnO2 spinel during the 100th cycle. It is remarkable that the addition of Bi2O3 in the nanosized β-MnO2 cathode exhibits outstanding capacity. After 100 dischargings, the battery demonstrates a capacity of 316 mA h g–1. Our findings can serve in the tailored cathode design in high capacity and rechargeable Zn/β-MnO2 alkaline batteries.
中文翻译:

纳米的嵌入和转化反应β-MnO的2阴极在次要的Zn / MnO的2碱性电池
这项工作报告可再充电的Zn /β-MnO的2个碱性电池作为有前途的固定储能。不同于具有差的循环性能的商业碱性电池中,纳米尺寸的β-MnO的2阴极在LiOH和KOH电解质的混合物能够以高容量可充电反应。揭开纳米β-MnO的的基本反应机制2,我们结合热力学框架与实验表征,包括电化学,X射线衍射和X射线光电子能谱。结果证明了一系列质子嵌入反应的(β-MnO的2 →γ-MnOOH的)和两相转化反应(γ-MnOOH的→锰(OH)2 →λ-MnO的2)所述第一周期和Li和H cointercalation在λ-MnO的的主机结构期间2的100中尖晶石次循环。值得注意的是,在添加Bi的2 Ó 3在纳米β-MnO的2阴极表现出优秀的能力。放电100次后,电池的容量为316 mA hg –1。我们的研究结果可以用于在高容量和可充电锌/β-MnO的修整阴极设计2碱性电池。
更新日期:2018-05-03
中文翻译:

纳米的嵌入和转化反应β-MnO的2阴极在次要的Zn / MnO的2碱性电池
这项工作报告可再充电的Zn /β-MnO的2个碱性电池作为有前途的固定储能。不同于具有差的循环性能的商业碱性电池中,纳米尺寸的β-MnO的2阴极在LiOH和KOH电解质的混合物能够以高容量可充电反应。揭开纳米β-MnO的的基本反应机制2,我们结合热力学框架与实验表征,包括电化学,X射线衍射和X射线光电子能谱。结果证明了一系列质子嵌入反应的(β-MnO的2 →γ-MnOOH的)和两相转化反应(γ-MnOOH的→锰(OH)2 →λ-MnO的2)所述第一周期和Li和H cointercalation在λ-MnO的的主机结构期间2的100中尖晶石次循环。值得注意的是,在添加Bi的2 Ó 3在纳米β-MnO的2阴极表现出优秀的能力。放电100次后,电池的容量为316 mA hg –1。我们的研究结果可以用于在高容量和可充电锌/β-MnO的修整阴极设计2碱性电池。











































 京公网安备 11010802027423号
京公网安备 11010802027423号