当前位置:
X-MOL 学术
›
J. Mater. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Descriptor of catalytic activity of metal sulfides for oxygen reduction reaction: a potential indicator for mineral flotation†
Journal of Materials Chemistry A ( IF 11.9 ) Pub Date : 2018-05-03 00:00:00 , DOI: 10.1039/c8ta01241e Hongbiao Tao 1, 2, 3, 4 , Subiao Liu 1, 2, 3, 4 , Jing-Li Luo 1, 2, 3, 4 , Phillip Choi 1, 2, 3, 4 , Qi Liu 1, 2, 3, 4 , Zhenghe Xu 1, 2, 3, 4
Journal of Materials Chemistry A ( IF 11.9 ) Pub Date : 2018-05-03 00:00:00 , DOI: 10.1039/c8ta01241e Hongbiao Tao 1, 2, 3, 4 , Subiao Liu 1, 2, 3, 4 , Jing-Li Luo 1, 2, 3, 4 , Phillip Choi 1, 2, 3, 4 , Qi Liu 1, 2, 3, 4 , Zhenghe Xu 1, 2, 3, 4
Affiliation
|
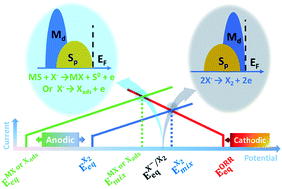
Froth flotation has been widely used to separate sulfide minerals from sulfide ores, where the oxygen reduction reaction (ORR) plays a significant role as the cathodic reaction. However, the intrinsic ORR mechanism over sulfide minerals and its effect on the flotation performance remain unclear. Herein, we explored the intrinsic ORR mechanism over metal sulfides through density functional theory (DFT) calculations along with the computational hydrogen electrode (CHE) model. Our results revealed that pyrite (FeS2) and chalcopyrite (CuFeS2) exhibited similar ORR behavior to that over platinum (Pt) but not galena (PbS) and sphalerite (ZnS). The relative computational overpotentials for the ORR were completely consistent with the experimental trend in the order of Pt < FeS2 < CuFeS2 < PbS < ZnS and linearly correlate with the oxygen binding energies. We thus conclude that the distinct ORR behaviors originate from the different oxygen binding strengths which are determined by the underlying electronic structure. More importantly, we demonstrated that the DFT calculated bulk centroids of the occupied S 3p band for metal sulfides strongly correlate with the experimentally measured rest potential of the counter sulfide minerals in xanthate solution, and consequently we established the descriptor–activity relationship. Using this relationship, we present that the mechanism underlying the mixed potential model regarding the xanthate–sulfide mineral interaction is essentially governed by the relative dominating role of the compositional metallic cation to the anionic sulfur in terms of the electronic structure around the Fermi level, which favors the formation of dixanthogen and metal-xanthate, respectively.
中文翻译:

金属硫化物对氧还原反应的催化活性的描述:矿物浮选的潜在指标†
泡沫浮选已广泛用于从硫化矿石中分离硫化物矿物,其中氧还原反应(ORR)作为阴极反应起着重要作用。但是,尚不清楚硫化物矿物的内在ORR机理及其对浮选性能的影响。本文中,我们通过密度泛函理论(DFT)计算以及计算性氢电极(CHE)模型探索了金属硫化物的固有ORR机理。我们的结果表明,黄铁矿(FeS 2)和黄铜矿(CuFeS 2)的ORR行为与铂(Pt)相似,但方铅矿(PbS)和闪锌矿(ZnS)却没有。ORR的相对计算超电势与实验趋势完全一致,按Pt <FeS 2的顺序<铜铁2<PbS <ZnS,并且与氧结合能线性相关。因此,我们得出结论,不同的ORR行为源自不同的氧结合强度,该结合强度由基础电子结构决定。更重要的是,我们证明了DFT计算出的金属硫化物占据的S 3p谱带的质心与黄药溶液中抗硫化物矿物的实验测得的静息电位密切相关,因此,我们建立了描述符-活性关系。利用这种关系,
更新日期:2018-05-03
中文翻译:

金属硫化物对氧还原反应的催化活性的描述:矿物浮选的潜在指标†
泡沫浮选已广泛用于从硫化矿石中分离硫化物矿物,其中氧还原反应(ORR)作为阴极反应起着重要作用。但是,尚不清楚硫化物矿物的内在ORR机理及其对浮选性能的影响。本文中,我们通过密度泛函理论(DFT)计算以及计算性氢电极(CHE)模型探索了金属硫化物的固有ORR机理。我们的结果表明,黄铁矿(FeS 2)和黄铜矿(CuFeS 2)的ORR行为与铂(Pt)相似,但方铅矿(PbS)和闪锌矿(ZnS)却没有。ORR的相对计算超电势与实验趋势完全一致,按Pt <FeS 2的顺序<铜铁2<PbS <ZnS,并且与氧结合能线性相关。因此,我们得出结论,不同的ORR行为源自不同的氧结合强度,该结合强度由基础电子结构决定。更重要的是,我们证明了DFT计算出的金属硫化物占据的S 3p谱带的质心与黄药溶液中抗硫化物矿物的实验测得的静息电位密切相关,因此,我们建立了描述符-活性关系。利用这种关系,



























 京公网安备 11010802027423号
京公网安备 11010802027423号