当前位置:
X-MOL 学术
›
Faraday Discuss.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Proton transfer in guanine–cytosine base pair analogues studied by NMR spectroscopy and PIMD simulations
Faraday Discussions ( IF 3.3 ) Pub Date : 2018-05-02 , DOI: 10.1039/c8fd00070k Radek Pohl 1, 2, 3 , Ondřej Socha 1, 2, 3 , Petr Slavíček 3, 4, 5, 6 , Michal Šála 1, 2, 3 , Paul Hodgkinson 7, 8, 9, 10 , Martin Dračínský 1, 2, 3
Faraday Discussions ( IF 3.3 ) Pub Date : 2018-05-02 , DOI: 10.1039/c8fd00070k Radek Pohl 1, 2, 3 , Ondřej Socha 1, 2, 3 , Petr Slavíček 3, 4, 5, 6 , Michal Šála 1, 2, 3 , Paul Hodgkinson 7, 8, 9, 10 , Martin Dračínský 1, 2, 3
Affiliation
|
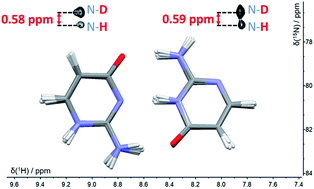
It has been hypothesised that proton tunnelling between paired nucleobases significantly enhances the formation of rare tautomeric forms and hence leads to errors in DNA replication. Here, we study nuclear quantum effects (NQEs) using deuterium isotope-induced changes of nitrogen NMR chemical shifts in a model base pair consisting of two tautomers of isocytosine, which form hydrogen-bonded dimers in the same way as the guanine–cytosine base pair. Isotope effects in NMR are consequences of NQEs, because ro-vibrational averaging of different isotopologues gives rise to different magnetic shielding of the nuclei. The experimental deuterium-induced chemical shift changes are compared with those calculated by a combination of path integral molecular dynamics (PIMD) simulations with DFT calculations of nuclear shielding. These calculations can directly link the observable isotope-induced shifts with NQEs. A comparison of the deuterium-induced changes of 15N chemical shifts with those predicted by PIMD simulations shows that inter-base proton transfer reactions do not take place in this system. We demonstrate, however, that NMR isotope shifts provide a unique possibility to study NQEs and to evaluate the accuracy of the computational methods used for modelling quantum effects in molecules. Calculations based on the PBE functional from the general-gradient-approximation family provided significantly worse predictions of deuterium isotope shifts than those with the hybrid B3LYP functional.
中文翻译:

核磁共振光谱法和PIMD模拟研究鸟嘌呤-胞嘧啶碱基对类似物中的质子转移
已经假设在成对的核碱基之间的质子隧穿显着增强了罕见的互变异构形式的形成,因此导致DNA复制中的错误。在这里,我们使用氘同位素诱导的氮NMR化学位移变化研究核量子效应(NQE),该模型碱基对由两个异胞嘧啶互变异构体组成,它们以与鸟嘌呤-胞嘧啶碱基对相同的方式形成氢键结合的二聚体。NMR中的同位素效应是NQE的结果,因为不同同位素的旋转振动平均会引起原子核的不同磁屏蔽。将氘引起的化学位移的实验变化与通过路径积分分子动力学(PIMD)模拟与核屏蔽的DFT计算相结合所计算出的化学位移变化进行比较。这些计算可以将可观测的同位素诱发的位移与NQE直接联系起来。氘引起的铁离子变化的比较PIMD模拟预测的化学位移为15 N,这表明该系统中未发生碱间质子转移反应。但是,我们证明了NMR同位素位移为研究NQE和评估用于模拟分子中量子效应的计算方法的准确性提供了独特的可能性。与基于杂化B3LYP的功能相比,基于一般梯度近似族的PBE功能的计算对氘同位素位移的预测要差得多。
更新日期:2018-12-21
中文翻译:

核磁共振光谱法和PIMD模拟研究鸟嘌呤-胞嘧啶碱基对类似物中的质子转移
已经假设在成对的核碱基之间的质子隧穿显着增强了罕见的互变异构形式的形成,因此导致DNA复制中的错误。在这里,我们使用氘同位素诱导的氮NMR化学位移变化研究核量子效应(NQE),该模型碱基对由两个异胞嘧啶互变异构体组成,它们以与鸟嘌呤-胞嘧啶碱基对相同的方式形成氢键结合的二聚体。NMR中的同位素效应是NQE的结果,因为不同同位素的旋转振动平均会引起原子核的不同磁屏蔽。将氘引起的化学位移的实验变化与通过路径积分分子动力学(PIMD)模拟与核屏蔽的DFT计算相结合所计算出的化学位移变化进行比较。这些计算可以将可观测的同位素诱发的位移与NQE直接联系起来。氘引起的铁离子变化的比较PIMD模拟预测的化学位移为15 N,这表明该系统中未发生碱间质子转移反应。但是,我们证明了NMR同位素位移为研究NQE和评估用于模拟分子中量子效应的计算方法的准确性提供了独特的可能性。与基于杂化B3LYP的功能相比,基于一般梯度近似族的PBE功能的计算对氘同位素位移的预测要差得多。











































 京公网安备 11010802027423号
京公网安备 11010802027423号