当前位置:
X-MOL 学术
›
RSC Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Isolation, expression and biochemical characterization of recombinant hyoscyamine-6β-hydroxylase from Brugmansia sanguinea – tuning the scopolamine production†
RSC Medicinal Chemistry ( IF 4.1 ) Pub Date : 2018-05-02 00:00:00 , DOI: 10.1039/c8md00090e Conrad Fischer 1, 2, 3, 4 , Moonhyuk Kwon 4, 5, 6, 7 , Dae-Kun Ro 4, 5, 6, 7 , Marco J. van Belkum 1, 2, 3, 4 , John C. Vederas 1, 2, 3, 4
RSC Medicinal Chemistry ( IF 4.1 ) Pub Date : 2018-05-02 00:00:00 , DOI: 10.1039/c8md00090e Conrad Fischer 1, 2, 3, 4 , Moonhyuk Kwon 4, 5, 6, 7 , Dae-Kun Ro 4, 5, 6, 7 , Marco J. van Belkum 1, 2, 3, 4 , John C. Vederas 1, 2, 3, 4
Affiliation
|
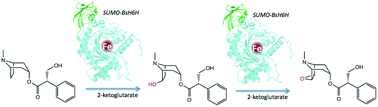
Hyoscyamine-6β-hydroxylase (H6H, EC 1.14.11.11) is a plant enzyme that catalyses the last two steps in the biosynthesis of the anticholinergic drug scopolamine, i.e. the hydroxylation of hyoscyamine to 6β-hydroxyhyoscyamine (anisodamine) and subsequent oxidative ring-closure to the 6,7-β-epoxide. A H6H gene homologue was isolated from the plant Brugmansia sanguinea (BsH6H) and recombinantly cloned into Escherichia coli, expressed and purified using an effective SUMO-fusion procedure. Enzymatic activity is approximately 40-fold higher for the first reaction step and the substrate affinity is comparable to other characterized H6H homologues (Km ∼ 60 μM). Truncation of an H6H enzyme flexible N-terminal region yields an active and stable yet more compact enzyme version.
中文翻译:

几内亚Brugmansia sanguinea 的重组hyscyamine-6β-羟化酶的分离,表达和生化特征–调节东pol碱的产量†
莨菪碱-6β羟化酶(H6H,EC 1.14.11.11)是一种植物酶,其催化在抗胆碱药物东莨菪碱的生物合成,最后两个步骤即莨菪碱的羟基化6β-hydroxyhyoscyamine(山莨菪碱)和随后的氧化环合生成6,7-β-环氧化物。甲H6H基因同源物是从植物中分离的木曼陀罗属荪(BsH6H)和重组克隆到大肠杆菌中,表达并使用有效SUMO-融合过程纯化。第一步反应的酶活性高约40倍,底物亲和力可与其他特征性H6H同源物(K m约60μM)。截短H6H酶的柔性N端区域可产生活性和稳定但更紧凑的酶版本。
更新日期:2018-05-02
中文翻译:

几内亚Brugmansia sanguinea 的重组hyscyamine-6β-羟化酶的分离,表达和生化特征–调节东pol碱的产量†
莨菪碱-6β羟化酶(H6H,EC 1.14.11.11)是一种植物酶,其催化在抗胆碱药物东莨菪碱的生物合成,最后两个步骤即莨菪碱的羟基化6β-hydroxyhyoscyamine(山莨菪碱)和随后的氧化环合生成6,7-β-环氧化物。甲H6H基因同源物是从植物中分离的木曼陀罗属荪(BsH6H)和重组克隆到大肠杆菌中,表达并使用有效SUMO-融合过程纯化。第一步反应的酶活性高约40倍,底物亲和力可与其他特征性H6H同源物(K m约60μM)。截短H6H酶的柔性N端区域可产生活性和稳定但更紧凑的酶版本。











































 京公网安备 11010802027423号
京公网安备 11010802027423号