当前位置:
X-MOL 学术
›
J. Mater. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
An outstanding effect of graphite in nano-MgH2–TiH2 on hydrogen storage performance†
Journal of Materials Chemistry A ( IF 11.9 ) Pub Date : 2018-05-02 00:00:00 , DOI: 10.1039/c8ta02969e Mykhaylo Lotoskyy 1, 2, 3, 4, 5 , Roman Denys 6, 7 , Volodymyr A. Yartys 7, 8 , Jon Eriksen 6, 7 , Jonathan Goh 1, 2, 3, 4, 5 , Serge Nyallang Nyamsi 1, 2, 3, 4, 5 , Cordellia Sita 1, 2, 3, 4, 5 , Franscious Cummings 3, 4, 5, 9
Journal of Materials Chemistry A ( IF 11.9 ) Pub Date : 2018-05-02 00:00:00 , DOI: 10.1039/c8ta02969e Mykhaylo Lotoskyy 1, 2, 3, 4, 5 , Roman Denys 6, 7 , Volodymyr A. Yartys 7, 8 , Jon Eriksen 6, 7 , Jonathan Goh 1, 2, 3, 4, 5 , Serge Nyallang Nyamsi 1, 2, 3, 4, 5 , Cordellia Sita 1, 2, 3, 4, 5 , Franscious Cummings 3, 4, 5, 9
Affiliation
|
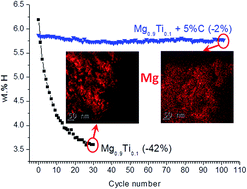
TiH2-modified MgH2 was prepared by high energy reactive ball milling (HRBM) of Mg and Ti in hydrogen and showed high weight H storage capacity and fast hydrogenation/dehydrogenation kinetics. However, a decrease in the reversible H storage capacity on cycling at high temperatures takes place and is a major obstacle for its use in hydrogen and heat storage applications. Reversible hydrogen absorption/desorption cycling of the materials requires use of the working temperature ≥330 °C and results in a partial step-by-step loss of the recoverable hydrogen storage capacity, with less significant changes in the rates of hydrogenation/dehydrogenation. After hydrogen desorption at 330–350 °C, hydrogen absorption can proceed at much lower temperatures, down to 24 °C. However, a significant decay in the reversible hydrogen capacity takes place with increasing number of cycles. The observed deterioration is caused by cycling-induced drastic morphological changes in the studied composite material leading to a segregation of TiH2 particles in the cycled samples instead of their initial homogeneous distribution. However, the introduction of 5 wt% of graphite into the MgH2–TiH2 composite system prepared by HRBM leads to an outstanding improvement of the hydrogen storage performance. Indeed, hydrogen absorption and desorption characteristics remain stable through 100 hydrogen absorption/desorption cycles and are related to an effect of the added graphite. The TEM study showed that carbon is uniformly distributed between the MgH2 grains covering segregated TiH2, preventing the grain growth and thus keeping the reversible storage capacity and the rates of hydrogen charge and discharge unchanged. Modelling of the kinetics of hydrogen absorption and desorption in the Mg–Ti and Mg–Ti–C composites showed that the reaction mechanisms significantly change depending on the presence or absence of graphite, the number of absorption–desorption cycles and the operating temperature.
中文翻译:

纳米MgH 2 -TiH 2中石墨对储氢性能的显着影响†
TiH 2改性的MgH 2Mg和Ti在氢气中通过高能反应球磨(HRBM)制备,具有高的储氢重量和快速的加氢/脱氢动力学。但是,高温循环时可逆储氢能力下降,这是其在氢气和储热应用中使用的主要障碍。材料的可逆氢吸收/解吸循环要求使用≥330°C的工作温度,并导致逐步恢复可回收氢存储容量的部分损失,而氢化/脱氢速率的变化较小。氢气在330–350°C下解吸后,氢气吸收可以在更低的温度(低至24°C)下进行。然而,随着循环次数的增加,可逆氢容量显着下降。观察到的劣化是由于循环诱导的复合材料剧烈形态变化导致TiH偏析引起的循环样品中有2个颗粒,而不是其初始均匀分布。但是,将5 wt%的石墨引入HRBM制备的MgH 2 -TiH 2复合体系中,可显着改善储氢性能。实际上,氢吸收和解吸特性在100个氢吸收/解吸循环中保持稳定,并且与添加的石墨的效果有关。TEM研究表明,碳均匀分布在覆盖偏析的TiH 2的MgH 2晶粒之间,防止晶粒长大,从而保持可逆存储容量和氢充放电速率不变。对Mg-Ti和Mg-Ti-C复合材料中氢吸收和解吸动力学的建模表明,反应机理会根据是否存在石墨,吸收-解吸循环数和操作温度而发生显着变化。
更新日期:2018-05-02
中文翻译:

纳米MgH 2 -TiH 2中石墨对储氢性能的显着影响†
TiH 2改性的MgH 2Mg和Ti在氢气中通过高能反应球磨(HRBM)制备,具有高的储氢重量和快速的加氢/脱氢动力学。但是,高温循环时可逆储氢能力下降,这是其在氢气和储热应用中使用的主要障碍。材料的可逆氢吸收/解吸循环要求使用≥330°C的工作温度,并导致逐步恢复可回收氢存储容量的部分损失,而氢化/脱氢速率的变化较小。氢气在330–350°C下解吸后,氢气吸收可以在更低的温度(低至24°C)下进行。然而,随着循环次数的增加,可逆氢容量显着下降。观察到的劣化是由于循环诱导的复合材料剧烈形态变化导致TiH偏析引起的循环样品中有2个颗粒,而不是其初始均匀分布。但是,将5 wt%的石墨引入HRBM制备的MgH 2 -TiH 2复合体系中,可显着改善储氢性能。实际上,氢吸收和解吸特性在100个氢吸收/解吸循环中保持稳定,并且与添加的石墨的效果有关。TEM研究表明,碳均匀分布在覆盖偏析的TiH 2的MgH 2晶粒之间,防止晶粒长大,从而保持可逆存储容量和氢充放电速率不变。对Mg-Ti和Mg-Ti-C复合材料中氢吸收和解吸动力学的建模表明,反应机理会根据是否存在石墨,吸收-解吸循环数和操作温度而发生显着变化。



























 京公网安备 11010802027423号
京公网安备 11010802027423号