当前位置:
X-MOL 学术
›
J. Chem. Educ.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Solubility from the Femtoscale to the Macroscale
Journal of Chemical Education ( IF 2.5 ) Pub Date : 2018-05-02 00:00:00 , DOI: 10.1021/acs.jchemed.7b00965 David W. Pollock 1 , Giovanna T. Truong 1 , Jessica L. Bonjour 2 , John A. Frost 3
Journal of Chemical Education ( IF 2.5 ) Pub Date : 2018-05-02 00:00:00 , DOI: 10.1021/acs.jchemed.7b00965 David W. Pollock 1 , Giovanna T. Truong 1 , Jessica L. Bonjour 2 , John A. Frost 3
Affiliation
|
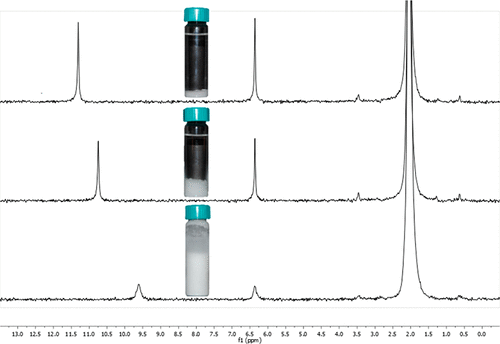
Solubility is frequently introduced at the high school and introductory college levels through the symbolic domain using net ionic equations and solubility product constants. Students may become proficient with spectator ion cancellation and skilled with algorithmic mathematical applications of solubility without obtaining a deeper understanding of the underlying concept of solubility. We present a convenient chemical system that allows for rapid data collection through benchtop nuclear magnetic resonance (NMR) spectroscopy and simple evaporation experiments. NMR spectroscopy can measure the particulate-level properties of hydrogen nuclei, objects on the order of a femtometer in size. Solvent evaporation to macroscopic solute residue ties solubility back to a visual frame of reference for students. Data collected from these dissimilar methods of measurement lend themselves to relating the particulate, macroscopic, and symbolic domains of understanding in a hands-on laboratory setting.
中文翻译:

从毫微尺度到宏观尺度的溶解度
溶解度经常在高中和大学入门级通过使用净离子方程式和溶解度乘积常数的符号域引入。学生可能会精通旁观者消除离子,并熟练掌握溶解度的算法数学应用,而无需深入了解溶解度的基本概念。我们提供了一种便捷的化学系统,可通过台式核磁共振(NMR)光谱和简单的蒸发实验快速收集数据。NMR光谱学可以测量氢核的颗粒级性质,物体的大小约为飞安特级。溶剂蒸发到宏观溶质残留物,将溶解度与学生的视觉参考系联系起来。
更新日期:2018-05-02
中文翻译:

从毫微尺度到宏观尺度的溶解度
溶解度经常在高中和大学入门级通过使用净离子方程式和溶解度乘积常数的符号域引入。学生可能会精通旁观者消除离子,并熟练掌握溶解度的算法数学应用,而无需深入了解溶解度的基本概念。我们提供了一种便捷的化学系统,可通过台式核磁共振(NMR)光谱和简单的蒸发实验快速收集数据。NMR光谱学可以测量氢核的颗粒级性质,物体的大小约为飞安特级。溶剂蒸发到宏观溶质残留物,将溶解度与学生的视觉参考系联系起来。











































 京公网安备 11010802027423号
京公网安备 11010802027423号