当前位置:
X-MOL 学术
›
Energy Environ. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A g-C3N4 based photoelectrochemical cell using O2/H2O redox couples†
Energy & Environmental Science ( IF 32.4 ) Pub Date : 2018-05-01 00:00:00 , DOI: 10.1039/c7ee03459h Naiyun Liu 1, 2, 3, 4, 5 , Mumei Han 1, 2, 3, 4, 5 , Yue Sun 1, 2, 3, 4, 5 , Cheng Zhu 1, 2, 3, 4, 5 , Yunjie Zhou 1, 2, 3, 4, 5 , Yalin Zhang 1, 2, 3, 4, 5 , Hui Huang 1, 2, 3, 4, 5 , Vladimir Kremnican 1, 2, 3, 4, 5 , Yang Liu 1, 2, 3, 4, 5 , Yeshayahu Lifshitz 1, 2, 3, 4, 5 , Zhenhui Kang 1, 2, 3, 4, 5
Energy & Environmental Science ( IF 32.4 ) Pub Date : 2018-05-01 00:00:00 , DOI: 10.1039/c7ee03459h Naiyun Liu 1, 2, 3, 4, 5 , Mumei Han 1, 2, 3, 4, 5 , Yue Sun 1, 2, 3, 4, 5 , Cheng Zhu 1, 2, 3, 4, 5 , Yunjie Zhou 1, 2, 3, 4, 5 , Yalin Zhang 1, 2, 3, 4, 5 , Hui Huang 1, 2, 3, 4, 5 , Vladimir Kremnican 1, 2, 3, 4, 5 , Yang Liu 1, 2, 3, 4, 5 , Yeshayahu Lifshitz 1, 2, 3, 4, 5 , Zhenhui Kang 1, 2, 3, 4, 5
Affiliation
|
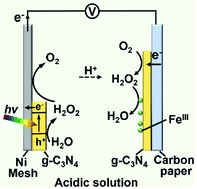
Utilization of solar energy for electric power production provides a practical approach for partially solving the energy crisis and at the same time reducing environmental pollution. Here, we demonstrate a photoelectrochemical cell based on a g-C3N4 photocatalyst using O2/H2O redox couples, fabricated with iron(III) phthalocyaninate [FeIII(Pc)Cl] mixed with g-C3N4 sprayed on carbon paper as the cathode and a nickel mesh (Ni mesh) coated with g-C3N4 as the anode. Under irradiation, g-C3N4 in the anode harvests photons and produces excited electrons and holes. The produced electrons transfer to the cathode by an external circuit and reduce O2 near the cathode to H2O2, electrochemically catalyzed by g-C3N4 through two-electron, two-proton processes, while the produced H2O2 is further reduced by [FeIII(Pc)Cl] to H2O. The generated holes in the anode oxidize H2O to H2O2 by two-electron, two-proton processes. The formed H2O2 can further be oxidized by the Ni mesh with O2 generation. This photoelectrochemical cell achieves an open-circuit voltage of 0.91 V under AM 1.5G solar light (1 sun, 100 mW cm−2) in 0.1 M HCl under air atmosphere. The total solar to electric power efficiency of this cell is 0.146%. Additionally this cell can generate and store H2O2 (chemical energy) with the electrodes disconnected under light, where water oxidation photocatalyzed by g-C3N4 will occur on both the electrodes. By connecting the electrodes, the cell can be operated as a fuel cell using H2O2 as the fuel in the absence of sunlight with an area-specific capacity of 350 mC cm−2 and a mass-specific capacity of 237 C g−1.
中文翻译:

使用O 2 / H 2 O氧化还原对的基于 gC 3 N 4的光电化学电池†
将太阳能用于电力生产为部分解决能源危机并同时减少环境污染提供了一种实用的方法。在这里,我们演示了一种基于O 2 / H 2 O氧化还原对的基于gC 3 N 4光催化剂的光电化学电池,该电池由混合有gC 3 N 4的酞菁铁(III)酞菁铁[Fe III(Pc)Cl]喷涂在复写纸上制成作为阴极,镍网(Ni网)涂覆有gC 3 N 4作为阳极。照射下,gC 3 N 4在阳极中收集光子并产生激发的电子和空穴。生成的电子通过外部电路转移到阴极,并将阴极附近的O 2还原为g 2 C 3 N 4通过双电子,两个质子过程进行电化学催化,而生成的H 2 O 2进一步被还原为H 2 O 2。通过的[Fe减少III(PC)CL]至H 2 O的产生的孔中的阳极氧化ħ 2 ö至H 2 ö 2由两电子,双质子过程。形成的H 2 O 2镍可以进一步被O 2生成的镍网氧化。该光电化学电池在空气气氛下,在0.1 M HCl中的AM 1.5G太阳光(1 sun,100 mW cm -2)下达到0.91 V的开路电压。该电池的总太阳能发电效率为0.146%。另外,该电池可在电极在光下断开的情况下生成并存储H 2 O 2(化学能),在此情况下,两个电极上均会发生被gC 3 N 4光催化的水氧化。通过连接电极,可以使用H 2 O 2将电池用作燃料电池作为没有阳光的燃料,面积比容量为350 mC cm -2,质量比容量为237 C g -1。
更新日期:2018-05-01
中文翻译:

使用O 2 / H 2 O氧化还原对的基于 gC 3 N 4的光电化学电池†
将太阳能用于电力生产为部分解决能源危机并同时减少环境污染提供了一种实用的方法。在这里,我们演示了一种基于O 2 / H 2 O氧化还原对的基于gC 3 N 4光催化剂的光电化学电池,该电池由混合有gC 3 N 4的酞菁铁(III)酞菁铁[Fe III(Pc)Cl]喷涂在复写纸上制成作为阴极,镍网(Ni网)涂覆有gC 3 N 4作为阳极。照射下,gC 3 N 4在阳极中收集光子并产生激发的电子和空穴。生成的电子通过外部电路转移到阴极,并将阴极附近的O 2还原为g 2 C 3 N 4通过双电子,两个质子过程进行电化学催化,而生成的H 2 O 2进一步被还原为H 2 O 2。通过的[Fe减少III(PC)CL]至H 2 O的产生的孔中的阳极氧化ħ 2 ö至H 2 ö 2由两电子,双质子过程。形成的H 2 O 2镍可以进一步被O 2生成的镍网氧化。该光电化学电池在空气气氛下,在0.1 M HCl中的AM 1.5G太阳光(1 sun,100 mW cm -2)下达到0.91 V的开路电压。该电池的总太阳能发电效率为0.146%。另外,该电池可在电极在光下断开的情况下生成并存储H 2 O 2(化学能),在此情况下,两个电极上均会发生被gC 3 N 4光催化的水氧化。通过连接电极,可以使用H 2 O 2将电池用作燃料电池作为没有阳光的燃料,面积比容量为350 mC cm -2,质量比容量为237 C g -1。









































 京公网安备 11010802027423号
京公网安备 11010802027423号