当前位置:
X-MOL 学术
›
J. Mater. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Alkaline earth metal oxide nanocluster modification of rutile TiO2 (110) promotes water activation and CO2 chemisorption†
Journal of Materials Chemistry A ( IF 10.7 ) Pub Date : 2018-05-01 00:00:00 , DOI: 10.1039/c8ta01789a Michael Nolan 1, 2, 3, 4
Journal of Materials Chemistry A ( IF 10.7 ) Pub Date : 2018-05-01 00:00:00 , DOI: 10.1039/c8ta01789a Michael Nolan 1, 2, 3, 4
Affiliation
|
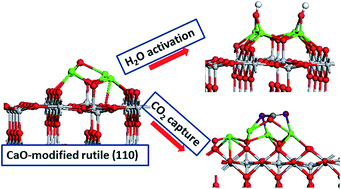
Metal oxide photocatalysts are widely studied for applications in solar driven environmental remediation, antimicrobial activity, hydrogen production and CO2 reduction to fuels. Common requirements for each technology include absorption of visible light, reduced charge carrier recombination and the ability to activate the initial molecule be it a pollutant, water or CO2. The leading photocatalyst is some form of TiO2. A significant amount of work has been undertaken to modifying TiO2 to induce visible light absorption. The structure and composition of the catalyst should facilitate separation of electrons and holes and having active sites on the catalyst is important to promote the initial adsorption and activation of molecules of interest. In this paper we present a first principles density functional theory (DFT) study of the modification of rutile TiO2 (110) with nanoclusters of the alkaline earth metal oxides (MgO, Ca, BaO) and we focus on the effect of surface modification on the key catalyst properties. The modification of rutile TiO2 with CaO and BaO induces a predicted red shift in light absorption. In all cases, photoexcited electrons and holes localise on oxygen in the nanocluster and surface Ti sites, thus enhancing charge separation. The presence of these non-bulk alkaline earth oxide nanoclusters provides highly active sites for water and CO2 adsorption. On MgO-rutile, water adsorbs molecularly and overcomes a barrier of only 0.36 eV for dissociation whereby hydroxyls are stabilised. On CaO- and BaO-modified rutile water adsorbs dissociatively. We attribute this to the high lying O 2p states in the alkaline earth oxide modifiers which are available to interact with water, as well as the non-bulk like geometry around the active site. Upon adsorption of CO2 the preferred binding mode is as a tridentate carbonate-like species, as characterised by geometry and vibrational modes. The carbonate is bound by up to 4 eV. Thus these heterostructures can be interesting for CO2 capture, helping alleviate the problem of CO2 emissions.
中文翻译:

金红石型TiO 2(110)的碱土金属氧化物纳米簇改性促进水活化和CO 2化学吸附†
对金属氧化物光催化剂进行了广泛的研究,以用于太阳能驱动的环境修复,抗菌活性,产氢和将CO 2还原为燃料。每种技术的共同要求包括吸收可见光,减少载流子复合以及激活初始分子(无论是污染物,水还是CO 2)的能力。主要的光催化剂是某些形式的TiO 2。已经进行了大量的工作来改性TiO 2引起可见光吸收。催化剂的结构和组成应有助于电子和空穴的分离,并且在催化剂上具有活性位点对促进目标分子的初始吸附和活化很重要。在本文中,我们提出了使用碱土金属氧化物(MgO,Ca,BaO)的纳米团簇修饰金红石TiO 2(110)的第一原理密度泛函理论(DFT)研究,并且我们重点研究了表面修饰对金红石TiO 2(110)的影响。关键的催化剂性能。金红石型TiO 2的改性CaO和BaO的掺杂会导致预计的光吸收红移。在所有情况下,光激发电子和空穴位于纳米团簇和表面Ti位置的氧上,从而增强了电荷分离。这些非本体的碱土金属氧化物纳米簇的存在为水和CO 2的吸附提供了高活性的位置。在MgO金红石上,水分子吸附并克服了仅0.36 eV的离解能使羟基稳定的障碍。在CaO和BaO改性的金红石上,水会解离地吸附。我们将其归因于可与水相互作用的碱土金属氧化物改性剂中较高的O 2p态,以及活性部位周围的非大块状几何形状。吸附CO 2时优选的结合模式是三齿碳酸盐样物质,其特征在于几何和振动模式。碳酸盐的结合力最高为4 eV。因此,这些异质结构对于捕获CO 2可能是令人感兴趣的,有助于缓解CO 2排放问题。
更新日期:2018-05-01
中文翻译:

金红石型TiO 2(110)的碱土金属氧化物纳米簇改性促进水活化和CO 2化学吸附†
对金属氧化物光催化剂进行了广泛的研究,以用于太阳能驱动的环境修复,抗菌活性,产氢和将CO 2还原为燃料。每种技术的共同要求包括吸收可见光,减少载流子复合以及激活初始分子(无论是污染物,水还是CO 2)的能力。主要的光催化剂是某些形式的TiO 2。已经进行了大量的工作来改性TiO 2引起可见光吸收。催化剂的结构和组成应有助于电子和空穴的分离,并且在催化剂上具有活性位点对促进目标分子的初始吸附和活化很重要。在本文中,我们提出了使用碱土金属氧化物(MgO,Ca,BaO)的纳米团簇修饰金红石TiO 2(110)的第一原理密度泛函理论(DFT)研究,并且我们重点研究了表面修饰对金红石TiO 2(110)的影响。关键的催化剂性能。金红石型TiO 2的改性CaO和BaO的掺杂会导致预计的光吸收红移。在所有情况下,光激发电子和空穴位于纳米团簇和表面Ti位置的氧上,从而增强了电荷分离。这些非本体的碱土金属氧化物纳米簇的存在为水和CO 2的吸附提供了高活性的位置。在MgO金红石上,水分子吸附并克服了仅0.36 eV的离解能使羟基稳定的障碍。在CaO和BaO改性的金红石上,水会解离地吸附。我们将其归因于可与水相互作用的碱土金属氧化物改性剂中较高的O 2p态,以及活性部位周围的非大块状几何形状。吸附CO 2时优选的结合模式是三齿碳酸盐样物质,其特征在于几何和振动模式。碳酸盐的结合力最高为4 eV。因此,这些异质结构对于捕获CO 2可能是令人感兴趣的,有助于缓解CO 2排放问题。











































 京公网安备 11010802027423号
京公网安备 11010802027423号