当前位置:
X-MOL 学术
›
Chem. Asian J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
C−H Alkenylation of Pyrroles by Electronically Matching Ligand Control
Chemistry - An Asian Journal ( IF 3.5 ) Pub Date : 2018-06-05 , DOI: 10.1002/asia.201800558 Hyun Tae Kim 1 , Woohyeong Lee 1 , Eunmin Kim 1 , Jung Min Joo 1
Chemistry - An Asian Journal ( IF 3.5 ) Pub Date : 2018-06-05 , DOI: 10.1002/asia.201800558 Hyun Tae Kim 1 , Woohyeong Lee 1 , Eunmin Kim 1 , Jung Min Joo 1
Affiliation
|
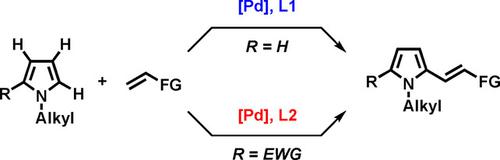
Directing group and substrate control strategies have frequently been employed for the regioselective C−H alkenylation of acid‐ and oxidant‐sensitive pyrrole heterocycles. We developed an undirected, aerobic strategy for the C−H alkenylation of N‐alkylpyrroles by ligand control. For C2‐alkenylation of electron‐rich N‐alkylpyrroles, an electrophilic palladium catalyst derived from Pd(OAc)2 and 4,5‐diazafluoren‐9‐one (DAF) was used. Alternatively, a combination of Pd(OAc)2 and a mono‐protected amino acid ligand, Ac‐Val‐OH, was useful for C5‐alkenylation of N‐alkylpyrroles possessing electron‐withdrawing groups at the C2 position. This approach based on the electronic effects of heterocycles and catalysts can rapidly provide a wide range of alkenyl pyrroles from readily available N‐alkylpyrroles and alkenes.
中文翻译:

电子匹配配体控制的吡咯的CH H烯基化
方向基和底物控制策略经常用于酸和氧化剂敏感的吡咯杂环的区域选择性CH烯基化。我们通过配体控制开发了N-烷基吡咯的C H烯基化的无向,好氧策略。对于富电子的N-烷基吡咯的C2-烯基化反应,使用了衍生自Pd(OAc)2和4,5-二氮杂芴-9-酮(DAF)的亲电钯催化剂。另外,Pd(OAc)2和单保护的氨基酸配体Ac-Val-OH的组合可用于N的C5-烯基化在C2位置具有吸电子基团的烷基吡咯。这种基于杂环和催化剂的电子效应的方法可以从容易获得的N烷基吡咯和烯烃中快速提供各种链烯基吡咯。
更新日期:2018-06-05
中文翻译:

电子匹配配体控制的吡咯的CH H烯基化
方向基和底物控制策略经常用于酸和氧化剂敏感的吡咯杂环的区域选择性CH烯基化。我们通过配体控制开发了N-烷基吡咯的C H烯基化的无向,好氧策略。对于富电子的N-烷基吡咯的C2-烯基化反应,使用了衍生自Pd(OAc)2和4,5-二氮杂芴-9-酮(DAF)的亲电钯催化剂。另外,Pd(OAc)2和单保护的氨基酸配体Ac-Val-OH的组合可用于N的C5-烯基化在C2位置具有吸电子基团的烷基吡咯。这种基于杂环和催化剂的电子效应的方法可以从容易获得的N烷基吡咯和烯烃中快速提供各种链烯基吡咯。











































 京公网安备 11010802027423号
京公网安备 11010802027423号