当前位置:
X-MOL 学术
›
Asian J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Disilylation of Palladacycles that were Generated through the C−H Activation of Aryl Halides
Asian Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2018-06-11 , DOI: 10.1002/ajoc.201800178 Xiaotian Ma 1 , Ailan Lu 1 , Xiaoming Ji 1 , Guangfa Shi 1 , Yanghui Zhang 1
Asian Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2018-06-11 , DOI: 10.1002/ajoc.201800178 Xiaotian Ma 1 , Ailan Lu 1 , Xiaoming Ji 1 , Guangfa Shi 1 , Yanghui Zhang 1
Affiliation
|
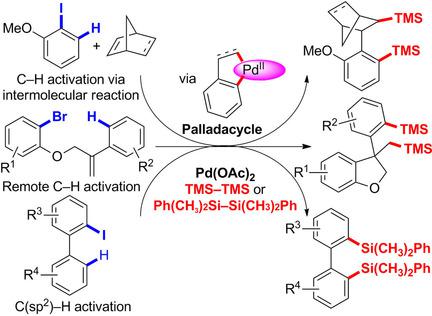
The reactions of various C,C‐palladacycles that were generated through the C−H activation of aryl halides with disilanes have been studied. The reaction of arylnorbornyl palladacycles, which were generated through a Catellani reaction, with hexamethyldisilane proceeded very efficiently to afford disilylated products. Palladacycles that were obtained through the remote C−H activation of aryl halides that contained a styrene moiety with an ether linkage also underwent a disilylation reaction with hexamethyldisilane. Furthermore, the reactions of dibenzopalladacyclopentadienes, which were generated from 2‐iodobiphenyls, with diphenyltetramethyldisilane were also investigated and a range of 2‐iodobiphenyls were disilylated in moderate yields.
中文翻译:

通过芳基卤化物的CH活化生成的Palladacycles的二异化
已经研究了通过C-H活化芳基卤化物与乙硅烷的反应生成的各种C,C-Palladacycles的反应。通过卡特拉尼反应生成的芳基降冰片基戊四环与六甲基二硅烷的反应非常有效地进行,从而得到二甲硅烷基化的产物。通过对含有带有醚键的苯乙烯部分的芳基卤化物进行远程CH活化而获得的Palladacycles也与六甲基乙硅烷发生了二甲硅烷基化反应。此外,还研究了由2-碘代联苯生成的二苯并四氢呋喃环戊二烯与二苯基四甲基二硅烷的反应,并以中等收率将一系列2-碘代联苯二甲苯化。
更新日期:2018-06-11
中文翻译:

通过芳基卤化物的CH活化生成的Palladacycles的二异化
已经研究了通过C-H活化芳基卤化物与乙硅烷的反应生成的各种C,C-Palladacycles的反应。通过卡特拉尼反应生成的芳基降冰片基戊四环与六甲基二硅烷的反应非常有效地进行,从而得到二甲硅烷基化的产物。通过对含有带有醚键的苯乙烯部分的芳基卤化物进行远程CH活化而获得的Palladacycles也与六甲基乙硅烷发生了二甲硅烷基化反应。此外,还研究了由2-碘代联苯生成的二苯并四氢呋喃环戊二烯与二苯基四甲基二硅烷的反应,并以中等收率将一系列2-碘代联苯二甲苯化。











































 京公网安备 11010802027423号
京公网安备 11010802027423号