Bioelectrochemistry ( IF 4.8 ) Pub Date : 2018-04-21 , DOI: 10.1016/j.bioelechem.2018.04.013 Javier Cervera , Alexis Pietak , Michael Levin , Salvador Mafe
|
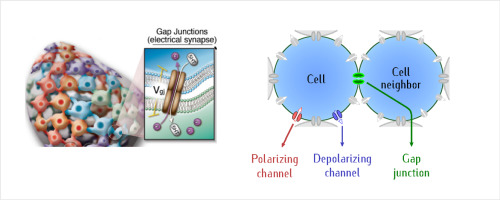
We review the basic concepts involved in bioelectrically-coupled multicellular domains, focusing on the role of membrane potentials (Vmem). In the first model, single-cell Vmem is modulated by two generic polarizing and depolarizing ion channels, while intercellular coupling is implemented via voltage-gated gap junctions. Biochemical and bioelectrical signals are integrated via a feedback loop between Vmem and the transcription and translation of a protein forming an ion channel. The effective rate constants depend on the single-cell Vmem because these potentials modulate the local concentrations of signaling molecules and ions. This electrochemically-based idealization of the complex biophysical problem suggests that the spatio-temporal map of single-cell potentials can influence downstream patterning processes by means of the voltage-gated gap junction interconnectivity, much as in the case of electronic devices where the control of electric potentials and currents allows the local modulation of the circuitry to achieve full functionality. An alternative theoretical approach, the BioElectrical Tissue Simulation Engine (BETSE), is also presented. The BETSE modeling environment utilizes finite volume techniques to simulate bioelectric states from the perspective of ion concentrations and fluxes. This model has been successfully applied to make predictions and explain experimental observations in a variety of embryonic, regenerative, and oncogenic contexts.
中文翻译:

间隙连接调节多细胞域中的生物电耦合:一种概念方法。
我们回顾了涉及生物电耦合的多细胞域的基本概念,重点是膜电位(V mem)的作用。在第一个模型中,单细胞V mem由两个通用的极化和去极化离子通道调节,而细胞间偶联则通过电压门控的间隙连接实现。生化和生物电信号通过V mem与形成离子通道的蛋白质的转录和翻译之间的反馈回路进行整合。有效速率常数取决于单电池V mem因为这些电势调节信号分子和离子的局部浓度。复杂生物物理问题的这种基于电化学的理想化表明,单细胞电势的时空图可以通过电压门控的间隙结互连性来影响下游的构图过程,就像电子设备控制电势和电流允许对电路进行局部调制,以实现全部功能。一种替代的理论方法,生物电动组织模拟引擎(BETSE),也被提出。BETSE建模环境利用有限体积技术从离子浓度和通量的角度模拟生物电状态。该模型已成功应用于在各种胚胎,再生和致癌环境中进行预测和解释实验观察。











































 京公网安备 11010802027423号
京公网安备 11010802027423号