当前位置:
X-MOL 学术
›
Phytochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Caudatan A, an undescribed human kidney-type glutaminase inhibitor with tetracyclic flavan from Ohwia caudata
Phytochemistry ( IF 3.2 ) Pub Date : 2018-08-01 , DOI: 10.1016/j.phytochem.2018.04.013 Yiwei Sun , Xiaohe Feng , Xuanli Liu , Cheng Qian , Xin Che , Fei Cao , Sanshan Jin , Dali Meng
Phytochemistry ( IF 3.2 ) Pub Date : 2018-08-01 , DOI: 10.1016/j.phytochem.2018.04.013 Yiwei Sun , Xiaohe Feng , Xuanli Liu , Cheng Qian , Xin Che , Fei Cao , Sanshan Jin , Dali Meng
|
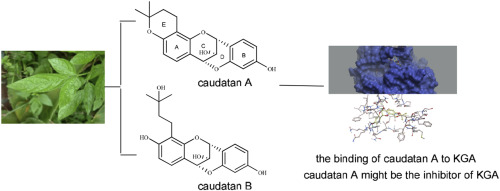
Human kidney-type glutaminase (KGA) is an important target that is often over expressed in many cancer cells but very few effective inhibitors of this enzyme have yet reached clinical trials. Caudatan A and caudatan B, two undescribed tetracyclic flavans with an unusual ether bond between the C-4 and C-2' were isolated from the roots of Ohwia caudata (Thunb.) H.Ohashi. Caudatan A exhibited stronger inhibitory activity and caudatan B showed moderate effect from the results of inhibitory activities evaluations on KGA. The molecular docking and primary structure-activity relationship analysis revealed that the less steric hindrance at ring A was necessary to the effect. Therefore, combined its better solubility than that of bis-2-(5-phenylacetimido-1,2,4-thiadiazol-2-yl)ethyl sulfide (BPTES), caudatan A might be the potential candidate as the inhibitor of KGA for further studies.
中文翻译:

Caudatan A,一种未描述的人肾型谷氨酰胺酶抑制剂,含有来自 Ohwia caudata 的四环黄烷
人肾型谷氨酰胺酶 (KGA) 是一个重要的靶标,通常在许多癌细胞中过度表达,但这种酶的有效抑制剂很少能达到临床试验。Caudatan A 和 caudatan B,两个未描述的四环黄烷,在 C-4 和 C-2' 之间具有不寻常的醚键,是从 Ohwia caudata (Thunb.) H.Ohashi 的根中分离出来的。从对KGA的抑制活性评估结果来看,尾坦A表现出更强的抑制活性,尾坦B表现出中等效果。分子对接和一级构效关系分析表明,A环的空间位阻较小是该效应所必需的。因此,结合其比双-2-(5-苯基乙酰亚胺-1,2,4-噻二唑-2-基)乙基硫醚(BPTES)更好的溶解性,
更新日期:2018-08-01
中文翻译:

Caudatan A,一种未描述的人肾型谷氨酰胺酶抑制剂,含有来自 Ohwia caudata 的四环黄烷
人肾型谷氨酰胺酶 (KGA) 是一个重要的靶标,通常在许多癌细胞中过度表达,但这种酶的有效抑制剂很少能达到临床试验。Caudatan A 和 caudatan B,两个未描述的四环黄烷,在 C-4 和 C-2' 之间具有不寻常的醚键,是从 Ohwia caudata (Thunb.) H.Ohashi 的根中分离出来的。从对KGA的抑制活性评估结果来看,尾坦A表现出更强的抑制活性,尾坦B表现出中等效果。分子对接和一级构效关系分析表明,A环的空间位阻较小是该效应所必需的。因此,结合其比双-2-(5-苯基乙酰亚胺-1,2,4-噻二唑-2-基)乙基硫醚(BPTES)更好的溶解性,










































 京公网安备 11010802027423号
京公网安备 11010802027423号