当前位置:
X-MOL 学术
›
ACS Med. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Discovery and Design of First Benzylamine-Based Ligands Binding to an Unlocked Conformation of the Complement Factor D
ACS Medicinal Chemistry Letters ( IF 3.5 ) Pub Date : 2018-04-24 00:00:00 , DOI: 10.1021/acsmedchemlett.8b00104 Anna Vulpetti 1 , Nils Ostermann 1 , Stefan Randl 1 , Taeyoung Yoon 1 , Aengus Mac Sweeney 1 , Frederic Cumin 1 , Edwige Lorthiois 1 , Simon Rüdisser 1 , Paul Erbel 1 , Jürgen Maibaum 1
ACS Medicinal Chemistry Letters ( IF 3.5 ) Pub Date : 2018-04-24 00:00:00 , DOI: 10.1021/acsmedchemlett.8b00104 Anna Vulpetti 1 , Nils Ostermann 1 , Stefan Randl 1 , Taeyoung Yoon 1 , Aengus Mac Sweeney 1 , Frederic Cumin 1 , Edwige Lorthiois 1 , Simon Rüdisser 1 , Paul Erbel 1 , Jürgen Maibaum 1
Affiliation
|
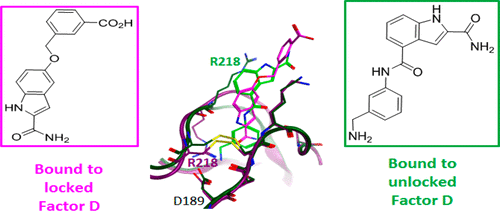
Complement Factor D, a serine protease of the S1 family and key component of the alternative pathway amplification loop, represents a promising target for the treatment of several prevalent and rare diseases linked to the innate immune system. Previously reported FD inhibitors have been shown to bind to the FD active site in its self-inhibited conformation characterized by the presence of a salt bridge at the bottom of the S1 pocket between Asp189 and Arg218. We report herein a new set of small-molecule FD ligands that harbor a basic S1 binding moiety directly binding to the carboxylate of Asp189, thereby displacing the Asp189-Arg218 ionic interaction and significantly changing the conformation of the self-inhibitory loop.
中文翻译:

发现和设计第一个基于苄胺的配体与补体因子D的解锁构象结合
补体因子D(S1家族的丝氨酸蛋白酶)和替代途径扩增环的关键组成部分,是治疗与先天免疫系统相关的几种流行和罕见疾病的有前途的靶标。先前报道的FD抑制剂已显示以其自抑制构象与FD活性位点结合,其特征是在Asp189和Arg218之间的S1口袋底部存在一个盐桥。我们在此报告了一组新的小分子FD配体,这些配体具有直接与Asp189的羧酸盐直接结合的基本S1结合部分,从而取代了Asp189-Arg218离子相互作用并显着改变了自抑制环的构象。
更新日期:2018-04-24
中文翻译:

发现和设计第一个基于苄胺的配体与补体因子D的解锁构象结合
补体因子D(S1家族的丝氨酸蛋白酶)和替代途径扩增环的关键组成部分,是治疗与先天免疫系统相关的几种流行和罕见疾病的有前途的靶标。先前报道的FD抑制剂已显示以其自抑制构象与FD活性位点结合,其特征是在Asp189和Arg218之间的S1口袋底部存在一个盐桥。我们在此报告了一组新的小分子FD配体,这些配体具有直接与Asp189的羧酸盐直接结合的基本S1结合部分,从而取代了Asp189-Arg218离子相互作用并显着改变了自抑制环的构象。









































 京公网安备 11010802027423号
京公网安备 11010802027423号