当前位置:
X-MOL 学术
›
Chem. Rev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Tetramethylpiperidine N-Oxyl (TEMPO), Phthalimide N-Oxyl (PINO), and Related N-Oxyl Species: Electrochemical Properties and Their Use in Electrocatalytic Reactions
Chemical Reviews ( IF 51.4 ) Pub Date : 2018-04-30 00:00:00 , DOI: 10.1021/acs.chemrev.7b00763 Jordan E. Nutting 1 , Mohammad Rafiee 1 , Shannon S. Stahl 1
Chemical Reviews ( IF 51.4 ) Pub Date : 2018-04-30 00:00:00 , DOI: 10.1021/acs.chemrev.7b00763 Jordan E. Nutting 1 , Mohammad Rafiee 1 , Shannon S. Stahl 1
Affiliation
|
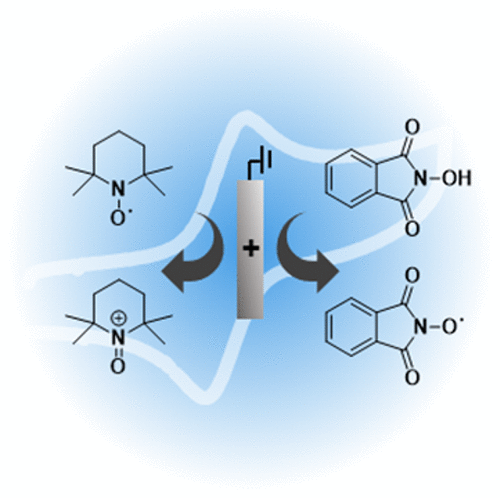
N-Oxyl compounds represent a diverse group of reagents that find widespread use as catalysts for the selective oxidation of organic molecules in both laboratory and industrial applications. While turnover of N-oxyl catalysts in oxidation reactions may be accomplished with a variety of stoichiometric oxidants, N-oxyl reagents have also been extensively used as catalysts under electrochemical conditions in the absence of chemical oxidants. Several classes of N-oxyl compounds undergo facile redox reactions at electrode surfaces, enabling them to mediate a wide range of electrosynthetic reactions. Electrochemical studies also provide insights into the structural properties and mechanisms of chemical and electrochemical catalysis by N-oxyl compounds. This review provides a comprehensive survey of the electrochemical properties and electrocatalytic applications of aminoxyls, imidoxyls, and related reagents, of which the two prototypical and widely used examples are 2,2,6,6-tetramethylpiperidine N-oxyl (TEMPO) and phthalimide N-oxyl (PINO).
中文翻译:

四甲基哌啶N-氧羰基(TEMPO),邻苯二甲酰亚胺N-氧羰基(PINO)和相关的N-氧羰基物种:电化学性质及其在电催化反应中的应用
N-羟基化合物代表了各种各样的试剂,它们在实验室和工业应用中被广泛用作有机分子选择性氧化的催化剂。尽管可以使用多种化学计量的氧化剂来完成氧化反应中N-氧基催化剂的转化,但是在不存在化学氧化剂的电化学条件下,N-氧基试剂也已被广泛用作催化剂。几类N-氧基化合物在电极表面容易发生氧化还原反应,使它们能够介导广泛的电合成反应。电化学研究还提供了有关N的化学和电化学催化的结构性质和机理的见解-氧基化合物。这篇综述提供了对氨氧基,亚胺氧基和相关试剂的电化学性质和电催化应用的全面调查,其中两个典型的且被广泛使用的例子是2,2,6,6-四甲基哌啶N-氧基(TEMPO)和邻苯二甲酰亚胺N -氧基(PINO)。
更新日期:2018-04-30
中文翻译:

四甲基哌啶N-氧羰基(TEMPO),邻苯二甲酰亚胺N-氧羰基(PINO)和相关的N-氧羰基物种:电化学性质及其在电催化反应中的应用
N-羟基化合物代表了各种各样的试剂,它们在实验室和工业应用中被广泛用作有机分子选择性氧化的催化剂。尽管可以使用多种化学计量的氧化剂来完成氧化反应中N-氧基催化剂的转化,但是在不存在化学氧化剂的电化学条件下,N-氧基试剂也已被广泛用作催化剂。几类N-氧基化合物在电极表面容易发生氧化还原反应,使它们能够介导广泛的电合成反应。电化学研究还提供了有关N的化学和电化学催化的结构性质和机理的见解-氧基化合物。这篇综述提供了对氨氧基,亚胺氧基和相关试剂的电化学性质和电催化应用的全面调查,其中两个典型的且被广泛使用的例子是2,2,6,6-四甲基哌啶N-氧基(TEMPO)和邻苯二甲酰亚胺N -氧基(PINO)。










































 京公网安备 11010802027423号
京公网安备 11010802027423号