当前位置:
X-MOL 学术
›
Small Methods
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Tuning the Electronic Spin State of Catalysts by Strain Control for Highly Efficient Water Electrolysis
Small Methods ( IF 10.7 ) Pub Date : 2018-03-25 , DOI: 10.1002/smtd.201800001 Shao-Hui Hsu,Sung-Fu Hung,Hsin-Yi Wang,Fang-Xing Xiao,Liping Zhang,Hongbin Yang,Hao Ming Chen,Jong-Min Lee,Bin Liu
Small Methods ( IF 10.7 ) Pub Date : 2018-03-25 , DOI: 10.1002/smtd.201800001 Shao-Hui Hsu,Sung-Fu Hung,Hsin-Yi Wang,Fang-Xing Xiao,Liping Zhang,Hongbin Yang,Hao Ming Chen,Jong-Min Lee,Bin Liu
|
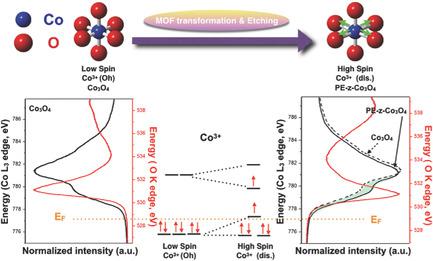
The electronic configuration is crucial in governing the binding strength of intermediates with catalysts, yet it is still challenging to control the catalysts' surface electronic spin state. Here, it is demonstrated that through surface metal–organic framework transformation followed by acid etching, the electronic spin state of surface Co3+ ions on spinel Co3O4 can be transformed from t2g6 to the high electronic spin state of t2g4eg2 by expanding the surface lattice constant, which significantly enhances the overlap of the eg orbital of cobalt with the oxygen adsorbates, and greatly improves the intermediates adsorption and thus the oxygen evolution reaction activity. The high electronic spin rich Co3O4 electrode exhibits an anodic current density of 10 mA cm−2 at an overpotential of 280 mV. The finding offers a rational design strategy to manipulate the electronic spin state of catalyst and the hybridization of molecular orbitals in water electrolysis.
中文翻译:

通过应变控制调节催化剂的电子自旋态以实现高效水电解
电子构型对于控制中间体与催化剂的结合强度至关重要,但是控制催化剂的表面电子自旋态仍然具有挑战性。在此表明,通过表面金属-有机骨架的转化以及随后的酸蚀刻,尖晶石Co 3 O 4上的表面Co 3+离子的电子自旋态可以从t 2g 6转变为高电子自旋态t 2g 4 e g 2通过扩展表面晶格常数,从而显着增强e g的重叠钴在轨道上的吸附有氧,大大提高了中间体的吸附性,从而提高了析氧反应的活性。高电子富自旋的Co 3 O 4电极在280 mV的过电势下表现出10 mA cm -2的阳极电流密度。该发现提供了合理的设计策略来控制催化剂的电子自旋态和水电解中分子轨道的杂化。
更新日期:2018-03-25
中文翻译:

通过应变控制调节催化剂的电子自旋态以实现高效水电解
电子构型对于控制中间体与催化剂的结合强度至关重要,但是控制催化剂的表面电子自旋态仍然具有挑战性。在此表明,通过表面金属-有机骨架的转化以及随后的酸蚀刻,尖晶石Co 3 O 4上的表面Co 3+离子的电子自旋态可以从t 2g 6转变为高电子自旋态t 2g 4 e g 2通过扩展表面晶格常数,从而显着增强e g的重叠钴在轨道上的吸附有氧,大大提高了中间体的吸附性,从而提高了析氧反应的活性。高电子富自旋的Co 3 O 4电极在280 mV的过电势下表现出10 mA cm -2的阳极电流密度。该发现提供了合理的设计策略来控制催化剂的电子自旋态和水电解中分子轨道的杂化。











































 京公网安备 11010802027423号
京公网安备 11010802027423号