当前位置:
X-MOL 学术
›
Electroanalysis
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Double Layer Impedance in Mixtures of Acetonitrile and Water
Electroanalysis ( IF 2.7 ) Pub Date : 2018-03-23 , DOI: 10.1002/elan.201800025 Koichi Jeremiah Aoki 1 , Jingyuan Chen 2 , Peng Tang 2
Electroanalysis ( IF 2.7 ) Pub Date : 2018-03-23 , DOI: 10.1002/elan.201800025 Koichi Jeremiah Aoki 1 , Jingyuan Chen 2 , Peng Tang 2
Affiliation
|
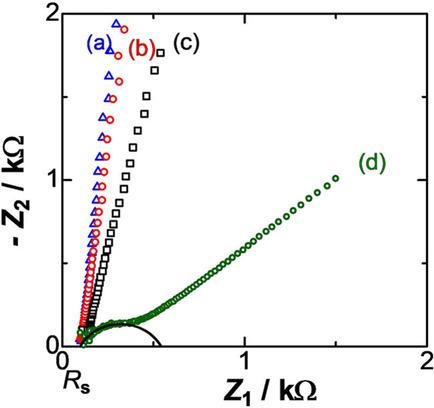
Double layer (DL) impedances were evaluated in mixed solutions of water and acetonitrile at various ratios in the polarized potential domain in order to find competitive orientation of the two solvent molecules on the platinum electrode. The DL capacitance at any ratio of the mixture shows the common power law of the ac‐frequency. The capacitance at molar fractions of water less than 0.2 increases linearly with the fraction, and reaches the value for aqueous solution. This variation indicates accumulation of water molecules on the electrode excluding acetonitrile molecules. It is modelled on the concept of the Langmuir‐type isotherm in which water and acetonitrile molecules have competitive interaction with the electrode. The experimental variations by the use of the isotherm yield the difference in the interaction energy, 6 kJ mol−1. The accumulation of water in the DL is supported by the formation of the adsorbed layer by insoluble ferrocene.
中文翻译:

乙腈和水的混合物中的双层阻抗
在水和乙腈的混合溶液中,在极化电势域中以不同比例评估了双层(DL)阻抗,以便找到两种溶剂分子在铂电极上的竞争取向。混合比例任意的DL电容都显示出交流频率的通用功率定律。水的摩尔分数小于0.2时的电容随分数线性增加,并达到水溶液的值。这种变化表明除乙腈分子外,水分子在电极上的积聚。它以Langmuir型等温线的概念为模型,其中水和乙腈分子与电极具有竞争性相互作用。通过使用等温线进行的实验变化产生了相互作用能的差异,为6 kJ mol-1。通过不溶性二茂铁形成吸附层来支持DL中水的积累。
更新日期:2018-03-23
中文翻译:

乙腈和水的混合物中的双层阻抗
在水和乙腈的混合溶液中,在极化电势域中以不同比例评估了双层(DL)阻抗,以便找到两种溶剂分子在铂电极上的竞争取向。混合比例任意的DL电容都显示出交流频率的通用功率定律。水的摩尔分数小于0.2时的电容随分数线性增加,并达到水溶液的值。这种变化表明除乙腈分子外,水分子在电极上的积聚。它以Langmuir型等温线的概念为模型,其中水和乙腈分子与电极具有竞争性相互作用。通过使用等温线进行的实验变化产生了相互作用能的差异,为6 kJ mol-1。通过不溶性二茂铁形成吸附层来支持DL中水的积累。











































 京公网安备 11010802027423号
京公网安备 11010802027423号