当前位置:
X-MOL 学术
›
Electroanalysis
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanistic Pathways and Identification of the Electrochemically Generated Oxidation Products of Flavonoid Eriodictyol in the Presence of Glutathione
Electroanalysis ( IF 2.7 ) Pub Date : 2018-04-06 , DOI: 10.1002/elan.201800071 Emad F. Newair 1 , Ayman Nafady 1, 2 , Refat Abdel-Hamid 1 , Abdullah M. Al-Enizi 2 , François Garcia 3
Electroanalysis ( IF 2.7 ) Pub Date : 2018-04-06 , DOI: 10.1002/elan.201800071 Emad F. Newair 1 , Ayman Nafady 1, 2 , Refat Abdel-Hamid 1 , Abdullah M. Al-Enizi 2 , François Garcia 3
Affiliation
|
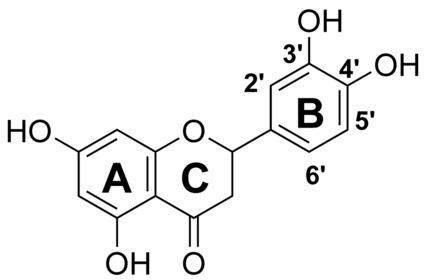
The metabolic oxidation pathways of dietary flavonoid eriodictyol (Er) are not very well-probed. In the present work, the electrochemical oxidation behavior of Er was studied in aqueous Britton-Robinson (B-R) buffer solution using cyclic voltammetry (CV), chronoamperometry (CA), and bulk-electrolysis (BE). The oxidation products and reaction pathways of Er in the absence and the presence of glutathione (GSH) were proposed and identified in view of the results obtained by ultra-high-performance liquid chromatography coupled with mass spectrometry (UPLC-MS). In the absence of GSH, eriodictyol shows one quasi-reversible oxidation process at E-1/2=0.305V, followed by a totally irreversible anodic peak at a more positive potential (E-p(a)=1.05V vs. Ag/AgCl, 3M KCl). Putatively, the first process corresponds to the oxidation of the catechol moiety on the B ring of Er while the second one is attributed to the oxidation of the resorcinol moiety on the A ring. In the presence of GSH, however, the anodic oxidation of Er was proposed to be an ECEC-type mechanism. The Er molecule first underwent a two-electron oxidation coupled with loss of two-proton to generate the corresponding quinone, which was either reduced to the original Er molecule by GSH, or further interacted with GSH to produce mono- and bi- glutathione conjugates of Er. The proposed mechanism was confirmed by digital simulation of the cyclic voltammograms.
中文翻译:

谷胱甘肽存在下黄酮圣草酚电化学生成氧化产物的机理途径及鉴定
膳食类黄酮圣草酚 (Er) 的代谢氧化途径尚未得到很好的探索。在目前的工作中,使用循环伏安法 (CV)、计时电流法 (CA) 和本体电解 (BE) 在布里顿-罗宾逊 (BR) 缓冲溶液中研究了 Er 的电化学氧化行为。结合超高效液相色谱-质谱联用(UPLC-MS)的结果,提出并鉴定了谷胱甘肽(GSH)不存在和存在下Er的氧化产物和反应途径。在不存在 GSH 的情况下,圣草酚在 E-1/2=0.305V 处显示出一种准可逆氧化过程,然后在更正的电位处出现完全不可逆的阳极峰(Ep(a)=1.05V vs. Ag/AgCl, 3M 氯化钾)。推定,第一个过程对应于 Er 的 B 环上儿茶酚部分的氧化,而第二个过程归因于 A 环上的间苯二酚部分的氧化。然而,在 GSH 存在下,Er 的阳极氧化被认为是一种 ECEC 型机制。Er 分子首先经历双电子氧化,同时失去两个质子,生成相应的醌,后者被 GSH 还原为原始 Er 分子,或进一步与 GSH 相互作用产生单谷胱甘肽和双谷胱甘肽共轭物。呃。通过循环伏安图的数字模拟证实了所提出的机制。Er 分子首先经历双电子氧化,同时失去两个质子,生成相应的醌,后者被 GSH 还原为原始 Er 分子,或进一步与 GSH 相互作用产生单谷胱甘肽和双谷胱甘肽共轭物。呃。通过循环伏安图的数字模拟证实了所提出的机制。Er 分子首先经历双电子氧化,同时失去两个质子,生成相应的醌,后者被 GSH 还原为原始的 Er 分子,或进一步与 GSH 相互作用产生单谷胱甘肽和双谷胱甘肽共轭物。呃。通过循环伏安图的数字模拟证实了所提出的机制。
更新日期:2018-04-06
中文翻译:

谷胱甘肽存在下黄酮圣草酚电化学生成氧化产物的机理途径及鉴定
膳食类黄酮圣草酚 (Er) 的代谢氧化途径尚未得到很好的探索。在目前的工作中,使用循环伏安法 (CV)、计时电流法 (CA) 和本体电解 (BE) 在布里顿-罗宾逊 (BR) 缓冲溶液中研究了 Er 的电化学氧化行为。结合超高效液相色谱-质谱联用(UPLC-MS)的结果,提出并鉴定了谷胱甘肽(GSH)不存在和存在下Er的氧化产物和反应途径。在不存在 GSH 的情况下,圣草酚在 E-1/2=0.305V 处显示出一种准可逆氧化过程,然后在更正的电位处出现完全不可逆的阳极峰(Ep(a)=1.05V vs. Ag/AgCl, 3M 氯化钾)。推定,第一个过程对应于 Er 的 B 环上儿茶酚部分的氧化,而第二个过程归因于 A 环上的间苯二酚部分的氧化。然而,在 GSH 存在下,Er 的阳极氧化被认为是一种 ECEC 型机制。Er 分子首先经历双电子氧化,同时失去两个质子,生成相应的醌,后者被 GSH 还原为原始 Er 分子,或进一步与 GSH 相互作用产生单谷胱甘肽和双谷胱甘肽共轭物。呃。通过循环伏安图的数字模拟证实了所提出的机制。Er 分子首先经历双电子氧化,同时失去两个质子,生成相应的醌,后者被 GSH 还原为原始 Er 分子,或进一步与 GSH 相互作用产生单谷胱甘肽和双谷胱甘肽共轭物。呃。通过循环伏安图的数字模拟证实了所提出的机制。Er 分子首先经历双电子氧化,同时失去两个质子,生成相应的醌,后者被 GSH 还原为原始的 Er 分子,或进一步与 GSH 相互作用产生单谷胱甘肽和双谷胱甘肽共轭物。呃。通过循环伏安图的数字模拟证实了所提出的机制。











































 京公网安备 11010802027423号
京公网安备 11010802027423号