当前位置:
X-MOL 学术
›
Energy Environ. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ni–Mo–O nanorod-derived composite catalysts for efficient alkaline water-to-hydrogen conversion via urea electrolysis†
Energy & Environmental Science ( IF 32.4 ) Pub Date : 2018-04-27 00:00:00 , DOI: 10.1039/c8ee00521d Zi-You Yu 1, 2, 3, 4, 5 , Chao-Chao Lang 1, 2, 3, 4, 5 , Min-Rui Gao 1, 2, 3, 4, 5 , Yu Chen 1, 2, 3, 4, 5 , Qi-Qi Fu 1, 2, 3, 4, 5 , Yu Duan 1, 2, 3, 4, 5 , Shu-Hong Yu 1, 2, 3, 4, 5
Energy & Environmental Science ( IF 32.4 ) Pub Date : 2018-04-27 00:00:00 , DOI: 10.1039/c8ee00521d Zi-You Yu 1, 2, 3, 4, 5 , Chao-Chao Lang 1, 2, 3, 4, 5 , Min-Rui Gao 1, 2, 3, 4, 5 , Yu Chen 1, 2, 3, 4, 5 , Qi-Qi Fu 1, 2, 3, 4, 5 , Yu Duan 1, 2, 3, 4, 5 , Shu-Hong Yu 1, 2, 3, 4, 5
Affiliation
|
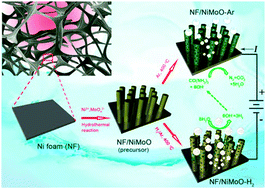
Photo/electrochemical splitting of water to hydrogen (H2) fuel is a sustainable way of meeting our energy demands at no environmental cost, but significant challenges remain: for example, the sluggish anodic reaction imposes a considerable overpotential requirement. By contrast, urea electrolysis offers the prospect of energy-saving H2 production together with urea-rich wastewater purification, whereas the lack of inexpensive and efficient urea oxidation reaction (UOR) catalysts places constraints on the development of this technique. Here we report a porous rod-like NiMoO4 with high oxidation states of the metal elements enabling highly efficient UOR electrocatalysis, which can be readily produced through annealing solid NiMoO4·xH2O as a starting precursor in Ar. This precursor gives the derived Ni/NiO/MoOx nanocomposite when switching the shielding gas from Ar to H2/Ar, exhibiting platinum-like activity for the hydrogen evolution reaction (HER) in alkaline electrolytes. Assembling an electrolytic cell using our developed UOR and HER catalysts as the anode and cathode can provide a current density of 10 milliamperes per square centimeter at a cell voltage of mere 1.38 volts, as well as remarkable operational stability, representing the best yet reported noble-metal-free urea electrolyser. Our results demonstrate the potential of nickel–molybdenum-based materials as efficient electrode catalysts for urea electrolysers that promises cost-effective and energy-saving H2 production.
中文翻译:

Ni-Mo-O纳米棒衍生的复合催化剂,可通过尿素电解有效地将碱水转化为氢†
将水光/电化学分解为氢(H 2)燃料是一种无偿环保的可持续方式,可满足我们的能源需求,但是仍然存在重大挑战:例如,缓慢的阳极反应提出了相当大的超电势要求。相比之下,尿素电解提供了节省H 2的生产以及富尿素废水净化的前景,而缺乏廉价,有效的尿素氧化反应(UOR)催化剂则对该技术的发展构成了限制。在这里,我们报告了一种多孔棒状NiMoO 4,其金属元素具有高氧化态,可以实现高效的UOR电催化,可以通过将固体NiMoO 4 ·x H 2 O作为Ar中的起始前体。当将保护气体从Ar转换为H 2 / Ar时,该前体产生了衍生的Ni / NiO / MoO x纳米复合材料,对碱性电解质中的氢释放反应(HER)表现出类似铂的活性。使用我们开发的UOR和HER催化剂作为阳极和阴极组装电解池,在仅1.38伏的电解池电压下可提供10毫安/平方厘米的电流密度,以及出色的工作稳定性,代表了迄今报道的最好的贵金属。无金属尿素电解槽。我们的结果证明了镍钼基材料作为尿素电解槽的高效电极催化剂的潜力,有望实现经济高效且节能的H2生产。
更新日期:2018-04-27
中文翻译:

Ni-Mo-O纳米棒衍生的复合催化剂,可通过尿素电解有效地将碱水转化为氢†
将水光/电化学分解为氢(H 2)燃料是一种无偿环保的可持续方式,可满足我们的能源需求,但是仍然存在重大挑战:例如,缓慢的阳极反应提出了相当大的超电势要求。相比之下,尿素电解提供了节省H 2的生产以及富尿素废水净化的前景,而缺乏廉价,有效的尿素氧化反应(UOR)催化剂则对该技术的发展构成了限制。在这里,我们报告了一种多孔棒状NiMoO 4,其金属元素具有高氧化态,可以实现高效的UOR电催化,可以通过将固体NiMoO 4 ·x H 2 O作为Ar中的起始前体。当将保护气体从Ar转换为H 2 / Ar时,该前体产生了衍生的Ni / NiO / MoO x纳米复合材料,对碱性电解质中的氢释放反应(HER)表现出类似铂的活性。使用我们开发的UOR和HER催化剂作为阳极和阴极组装电解池,在仅1.38伏的电解池电压下可提供10毫安/平方厘米的电流密度,以及出色的工作稳定性,代表了迄今报道的最好的贵金属。无金属尿素电解槽。我们的结果证明了镍钼基材料作为尿素电解槽的高效电极催化剂的潜力,有望实现经济高效且节能的H2生产。









































 京公网安备 11010802027423号
京公网安备 11010802027423号