当前位置:
X-MOL 学术
›
J. Proteome Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mass-Spectrometry-Based Identification of Cross-Links in Proteins Exposed to Photo-Oxidation and Peroxyl Radicals Using 18O Labeling and Optimized Tandem Mass Spectrometry Fragmentation
Journal of Proteome Research ( IF 3.8 ) Pub Date : 2018-04-26 , DOI: 10.1021/acs.jproteome.7b00881 Michele Mariotti 1 , Fabian Leinisch 2 , Diana Julie Leeming 3 , Birte Svensson 1 , Michael J. Davies 2 , Per Hägglund 1, 2
Journal of Proteome Research ( IF 3.8 ) Pub Date : 2018-04-26 , DOI: 10.1021/acs.jproteome.7b00881 Michele Mariotti 1 , Fabian Leinisch 2 , Diana Julie Leeming 3 , Birte Svensson 1 , Michael J. Davies 2 , Per Hägglund 1, 2
Affiliation
|
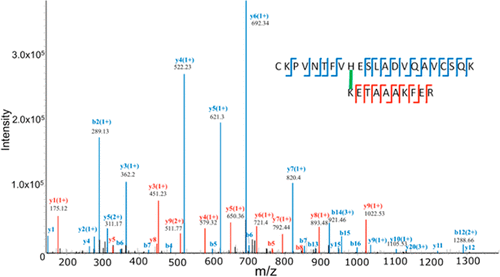
Protein cross-links are formed in regulated biochemical processes in many biological systems, but they are also generated inadvertently via the reactions of exogenous or endogenous oxidants. Site-specific identification and characterization of such cross-links is challenging, and the goal was, therefore, to develop mass-spectrometry-based approaches tailored for proteins subjected to oxidative challenges that also are applicable for the analysis of complex samples. Using trypsin-mediated 18O isotopic labeling, different types of data acquisition workflows, and designated database software tools, we successfully identified tyrosine–tyrosine, tyrosine–tryptophan, tyrosine–lysine, and histidine–lysine cross-links in proteins subjected to sensitizer-mediated photo-oxidation with rose bengal or chemical oxidation with peroxyl radicals generated from the water-soluble compound 2,2′-azobis(2-amidinopropane) dihydrochloride (AAPH). Subsequently, AAPH was also applied to a protein extract from the Gram-positive bacterium Lactococcus lactis, demonstrating the feasibility to identify tyrosine–tyrosine, tyrosine–tryptophan, and tryptophan–tryptophan cross-linked peptides in a complex system. Different fragmentation techniques were evaluated, and it was observed that higher-energy collisional dissociation (HCD) resulted in a higher number of identified cross-link peptides, while electron-transfer dissociation supplemented with HCD (EThcD) generally provides higher fragment ion coverage of the cross-linked peptides.
中文翻译:

基于质谱的交叉键识别中暴露于光氧化和过氧自由基的蛋白质中的交联,使用18 O标记和优化的串联质谱碎片化
蛋白质交联是在许多生物系统中受调节的生化过程中形成的,但它们也是通过外源性或内源性氧化剂的反应无意中产生的。这种交联的位点特异性鉴定和表征具有挑战性,因此目标是开发基于质谱的方法,该方法专为遭受氧化挑战的蛋白质量身定制,该方法也适用于复杂样品的分析。使用胰蛋白酶介导的18通过同位素标记,不同类型的数据采集工作流程以及指定的数据库软件工具,我们成功地鉴定了在敏化剂介导的光氧化作用下蛋白质中的酪氨酸-酪氨酸,酪氨酸-色氨酸,酪氨酸-赖氨酸和组氨酸-赖氨酸交联。由水溶性化合物2,2'-偶氮二(2-ami基丙烷)二盐酸盐(AAPH)产生的过氧自由基对玫瑰红进行孟加拉或化学氧化。随后,还将AAPH应用于革兰氏阳性细菌乳酸乳球菌的蛋白质提取物中证明了在复杂系统中鉴定酪氨酸-酪氨酸,酪氨酸-色氨酸和色氨酸-色氨酸交联肽的可行性。评估了不同的裂解技术,发现高能碰撞离解(HCD)导致鉴定出更多的交联肽,而补充了HCD(EThcD)的电子转移离解通常提供更高的碎片离子覆盖率。交联的肽。
更新日期:2018-04-27
中文翻译:

基于质谱的交叉键识别中暴露于光氧化和过氧自由基的蛋白质中的交联,使用18 O标记和优化的串联质谱碎片化
蛋白质交联是在许多生物系统中受调节的生化过程中形成的,但它们也是通过外源性或内源性氧化剂的反应无意中产生的。这种交联的位点特异性鉴定和表征具有挑战性,因此目标是开发基于质谱的方法,该方法专为遭受氧化挑战的蛋白质量身定制,该方法也适用于复杂样品的分析。使用胰蛋白酶介导的18通过同位素标记,不同类型的数据采集工作流程以及指定的数据库软件工具,我们成功地鉴定了在敏化剂介导的光氧化作用下蛋白质中的酪氨酸-酪氨酸,酪氨酸-色氨酸,酪氨酸-赖氨酸和组氨酸-赖氨酸交联。由水溶性化合物2,2'-偶氮二(2-ami基丙烷)二盐酸盐(AAPH)产生的过氧自由基对玫瑰红进行孟加拉或化学氧化。随后,还将AAPH应用于革兰氏阳性细菌乳酸乳球菌的蛋白质提取物中证明了在复杂系统中鉴定酪氨酸-酪氨酸,酪氨酸-色氨酸和色氨酸-色氨酸交联肽的可行性。评估了不同的裂解技术,发现高能碰撞离解(HCD)导致鉴定出更多的交联肽,而补充了HCD(EThcD)的电子转移离解通常提供更高的碎片离子覆盖率。交联的肽。









































 京公网安备 11010802027423号
京公网安备 11010802027423号