Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The potential of zwitterionic nanoliposomes against neurotoxic alpha-synuclein aggregates in Parkinson's Disease†
Nanoscale ( IF 5.8 ) Pub Date : 2018-04-26 00:00:00 , DOI: 10.1039/c8nr00632f Farhang Aliakbari 1, 2, 3, 4, 5 , Hossein Mohammad-Beigi 1, 2, 3, 4, 5 , Nasrollah Rezaei-Ghaleh 6, 7, 8 , Stefan Becker 8, 9, 10, 11 , Faezeh Dehghani Esmatabad 1, 2, 3, 4, 5 , Hadieh Alsadat Eslampanah Seyedi 1, 2, 3, 4, 5 , Hassan Bardania 5, 12, 13, 14 , Amir Tayaranian Marvian 1, 2, 3, 4, 5 , Joanna F. Collingwood 15, 16, 17, 18 , Gunna Christiansen 19, 20, 21, 22 , Markus Zweckstetter 6, 7, 8, 9, 10 , Daniel E. Otzen 20, 22, 23, 24 , Dina Morshedi 1, 2, 3, 4, 5
Nanoscale ( IF 5.8 ) Pub Date : 2018-04-26 00:00:00 , DOI: 10.1039/c8nr00632f Farhang Aliakbari 1, 2, 3, 4, 5 , Hossein Mohammad-Beigi 1, 2, 3, 4, 5 , Nasrollah Rezaei-Ghaleh 6, 7, 8 , Stefan Becker 8, 9, 10, 11 , Faezeh Dehghani Esmatabad 1, 2, 3, 4, 5 , Hadieh Alsadat Eslampanah Seyedi 1, 2, 3, 4, 5 , Hassan Bardania 5, 12, 13, 14 , Amir Tayaranian Marvian 1, 2, 3, 4, 5 , Joanna F. Collingwood 15, 16, 17, 18 , Gunna Christiansen 19, 20, 21, 22 , Markus Zweckstetter 6, 7, 8, 9, 10 , Daniel E. Otzen 20, 22, 23, 24 , Dina Morshedi 1, 2, 3, 4, 5
Affiliation
|
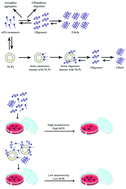
The protein α-synuclein (αSN) aggregates to form fibrils in neuronal cells of Parkinson's patients. Here we report on the effect of neutral (zwitterionic) nanoliposomes (NLPs), supplemented with cholesterol (NLP-Chol) and decorated with PEG (NLP-Chol-PEG), on αSN aggregation and neurotoxicity. Both NLPs retard αSN fibrillization in a concentration-independent fashion. They do so largely by increasing lag time (formation of fibrillization nuclei) rather than elongation (extension of existing nuclei). Interactions between neutral NLPs and αSN may locate to the N-terminus of the protein. This interaction can even perturb the interaction of αSN with negatively charged NLPs which induces an α-helical structure in αSN. This interaction was found to occur throughout the fibrillization process. Both NLP-Chol and NLP-Chol-PEG were shown to be biocompatible in vitro, and to reduce αSN neurotoxicity and reactive oxygen species (ROS) levels with no influence on intracellular calcium in neuronal cells, emphasizing a prospective role for NLPs in reducing αSN pathogenicity in vivo as well as utility as a vehicle for drug delivery.
中文翻译:

两性离子纳米脂质体对抗帕金森氏病中神经毒性α-突触核蛋白聚集体的潜力†
帕金森氏病患者的神经元细胞中的蛋白质α-突触核蛋白(αSN)聚集形成原纤维。在这里,我们报告了中性(两性离子)纳米脂质体(NLP),补充有胆固醇(NLP-Chol)并用PEG(NLP-Chol-PEG)装饰,对αSN聚集和神经毒性的影响。两种NLP均以浓度独立的方式阻滞αSN原纤维化。它们在很大程度上是通过增加滞后时间(原纤化核的形成)而不是延长(延长现有核的延伸)来实现的。中性NLP和αSN之间的相互作用可能位于蛋白质的N末端。这种相互作用甚至可以干扰αSN与带负电荷的NLP的相互作用,从而在αSN中诱导出α螺旋结构。发现这种相互作用发生在整个原纤化过程中。在体外,并减少αSN神经毒性和反应性氧物质(ROS)水平与神经元细胞中的胞内钙没有影响,强调减少αSN致病性NLPs预期作用在体内以及可用作用于药物递送的载体。
更新日期:2018-04-26
中文翻译:

两性离子纳米脂质体对抗帕金森氏病中神经毒性α-突触核蛋白聚集体的潜力†
帕金森氏病患者的神经元细胞中的蛋白质α-突触核蛋白(αSN)聚集形成原纤维。在这里,我们报告了中性(两性离子)纳米脂质体(NLP),补充有胆固醇(NLP-Chol)并用PEG(NLP-Chol-PEG)装饰,对αSN聚集和神经毒性的影响。两种NLP均以浓度独立的方式阻滞αSN原纤维化。它们在很大程度上是通过增加滞后时间(原纤化核的形成)而不是延长(延长现有核的延伸)来实现的。中性NLP和αSN之间的相互作用可能位于蛋白质的N末端。这种相互作用甚至可以干扰αSN与带负电荷的NLP的相互作用,从而在αSN中诱导出α螺旋结构。发现这种相互作用发生在整个原纤化过程中。在体外,并减少αSN神经毒性和反应性氧物质(ROS)水平与神经元细胞中的胞内钙没有影响,强调减少αSN致病性NLPs预期作用在体内以及可用作用于药物递送的载体。











































 京公网安备 11010802027423号
京公网安备 11010802027423号