当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Facile Epoxide Aminolysis Promoted by (t-BuO)2Mg and Its Application to the Synthesis of Efinaconazole
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2018-04-26 00:00:00 , DOI: 10.1021/acs.oprd.8b00081 Fuqiang Zhu 1, 2 , Yuanchao Xie 3 , Jian Zhang 4 , Guanghui Tian 4 , Hongjian Qin 4 , Xiaojun Yang 4 , Tianwen Hu 4 , Yang He 3 , Haji A. Aisa 1 , Jingshan Shen 3
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2018-04-26 00:00:00 , DOI: 10.1021/acs.oprd.8b00081 Fuqiang Zhu 1, 2 , Yuanchao Xie 3 , Jian Zhang 4 , Guanghui Tian 4 , Hongjian Qin 4 , Xiaojun Yang 4 , Tianwen Hu 4 , Yang He 3 , Haji A. Aisa 1 , Jingshan Shen 3
Affiliation
|
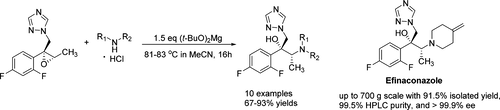
A novel and efficient method for the aminolysis of trizole epoxides is described. This method consists of a facile ring opening of the epoxides mediated by t-BuOMgCl generated in situ from amine hydrochlorides and (t-BuO)2Mg. The desired β-amino alcohols were obtained in good yields without employing other heavy metals or precious catalysts. The optimized conditions were successfully applied to the synthesis of a number of potential triazole antifungal compounds as well as efinaconazole on up to a 700 g scale.
中文翻译:

(t -BuO)2 Mg促进的环氧胺的简便水解及其在合成芬太康唑中的应用。
描述了一种新颖和有效的三唑环氧化物氨解的方法。该方法包括由胺盐酸盐和(t- BuO)2 Mg原位生成的t- BuOMgCl介导的环氧化物的容易的开环。在不使用其他重金属或贵重催化剂的情况下,以良好的产率获得了所需的β-氨基醇。优化的条件已成功地用于合成许多潜在的三唑类抗真菌化合物以及efinaconazole(最大量为700 g)。
更新日期:2018-04-26
中文翻译:

(t -BuO)2 Mg促进的环氧胺的简便水解及其在合成芬太康唑中的应用。
描述了一种新颖和有效的三唑环氧化物氨解的方法。该方法包括由胺盐酸盐和(t- BuO)2 Mg原位生成的t- BuOMgCl介导的环氧化物的容易的开环。在不使用其他重金属或贵重催化剂的情况下,以良好的产率获得了所需的β-氨基醇。优化的条件已成功地用于合成许多潜在的三唑类抗真菌化合物以及efinaconazole(最大量为700 g)。











































 京公网安备 11010802027423号
京公网安备 11010802027423号