当前位置:
X-MOL 学术
›
Cryst. Growth Des.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Inhibition of Nucleation Using a Dilute, Weakly Hydrogen-Bonding Molecular Additive
Crystal Growth & Design ( IF 3.2 ) Pub Date : 2018-04-24 00:00:00 , DOI: 10.1021/acs.cgd.8b00367 Carlos A. Pons Siepermann 1, 2 , Allan S. Myerson 1, 2
Crystal Growth & Design ( IF 3.2 ) Pub Date : 2018-04-24 00:00:00 , DOI: 10.1021/acs.cgd.8b00367 Carlos A. Pons Siepermann 1, 2 , Allan S. Myerson 1, 2
Affiliation
|
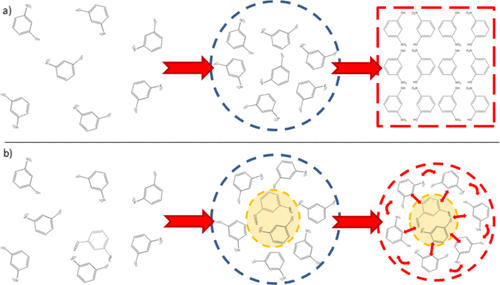
The effect of a weakly interacting dilute complexing agent on the nucleation rates of a small-molecule solute was explored using the model system of 3-nitrophenol as the inhibited molecule in a toluene solution with a 3-aminobenzoic acid inhibitor. Induction times were measured experimentally using the probability distribution of the solute crystals nucleation events as a function of time. Experimental results demonstrated that a small concentration of inhibitor (0.25% molar with respect to solute) led to a 230% increase in induction times with respect to noninhibited controls at identical supersaturation. Product crystal growth rates, polymorphism, and purity were found to be unaffected by the complexing agent, confirming that the change in nucleation rate was only due to nucleation inhibition. The nucleation rate kinetics of the solute were studied as a function of supersaturation, with and without the inhibitor. Data indicated that upon the addition of the inhibitor, there is a sharp decrease in the pre-exponential factor for the nucleation rate correlation, while there is minimal change on the activation energy. The experimental data were rationalized using a nucleation kinetics model based on the two-step nucleation theory. Analysis of the parameters defined in the nucleation rate equation for the two-step model indicated that the change in rates came from suppression of the ordering kinetic constant for the transition from a prenucleation cluster to a nucleus. A mechanism for the inhibition was proposed in which the formation of intermolecular complexes between solute and additive disrupts the ordering step by hindering the rearrangement of molecules within clusters.
中文翻译:

使用稀弱键合氢的分子添加剂抑制成核
使用3-硝基苯酚作为受抑制分子在带有3-氨基苯甲酸抑制剂的甲苯溶液中的模型系统,探索了弱相互作用的稀释络合剂对小分子溶质成核速率的影响。使用溶质晶体成核事件的概率分布作为时间的函数,通过实验测量了诱导时间。实验结果表明,在相同的过饱和度下,低浓度的抑制剂(相对于溶质的摩尔浓度为0.25%)导致诱导时间增加,相对于非抑制的对照而言,诱导时间增加了230%。发现产物晶体的生长速率,多态性和纯度不受络合剂的影响,证实成核速率的变化仅是由于成核抑制所致。在有和没有抑制剂的情况下,研究了溶质的成核速率动力学与过饱和的关系。数据表明,加入抑制剂后,成核速率相关性的指数前因子急剧下降,而活化能的变化却很小。使用基于两步成核理论的成核动力学模型合理化了实验数据。对两步模型的成核速率方程中定义的参数的分析表明,速率的变化来自抑制从预成核簇到核的跃迁的有序动力学常数。
更新日期:2018-04-24
中文翻译:

使用稀弱键合氢的分子添加剂抑制成核
使用3-硝基苯酚作为受抑制分子在带有3-氨基苯甲酸抑制剂的甲苯溶液中的模型系统,探索了弱相互作用的稀释络合剂对小分子溶质成核速率的影响。使用溶质晶体成核事件的概率分布作为时间的函数,通过实验测量了诱导时间。实验结果表明,在相同的过饱和度下,低浓度的抑制剂(相对于溶质的摩尔浓度为0.25%)导致诱导时间增加,相对于非抑制的对照而言,诱导时间增加了230%。发现产物晶体的生长速率,多态性和纯度不受络合剂的影响,证实成核速率的变化仅是由于成核抑制所致。在有和没有抑制剂的情况下,研究了溶质的成核速率动力学与过饱和的关系。数据表明,加入抑制剂后,成核速率相关性的指数前因子急剧下降,而活化能的变化却很小。使用基于两步成核理论的成核动力学模型合理化了实验数据。对两步模型的成核速率方程中定义的参数的分析表明,速率的变化来自抑制从预成核簇到核的跃迁的有序动力学常数。











































 京公网安备 11010802027423号
京公网安备 11010802027423号