当前位置:
X-MOL 学术
›
Sustain. Energy Fuels
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Highly dispersed and disordered nickel–iron layered hydroxides and sulphides: robust and high-activity water oxidation catalysts†
Sustainable Energy & Fuels ( IF 5.6 ) Pub Date : 2018-04-25 00:00:00 , DOI: 10.1039/c8se00129d Manjunath Chatti 1, 2, 2, 3, 4 , Alexey M. Glushenkov 3, 5, 6, 7 , Thomas Gengenbach 3, 8, 9 , Gregory P. Knowles 1, 2, 3 , Tiago C. Mendes 1, 2, 3 , Amanda V. Ellis 3, 5, 6, 7 , Leone Spiccia 1, 2, 2, 3, 4 , Rosalie K. Hocking 3, 10, 11 , Alexandr N. Simonov 1, 2, 2, 3, 4
Sustainable Energy & Fuels ( IF 5.6 ) Pub Date : 2018-04-25 00:00:00 , DOI: 10.1039/c8se00129d Manjunath Chatti 1, 2, 2, 3, 4 , Alexey M. Glushenkov 3, 5, 6, 7 , Thomas Gengenbach 3, 8, 9 , Gregory P. Knowles 1, 2, 3 , Tiago C. Mendes 1, 2, 3 , Amanda V. Ellis 3, 5, 6, 7 , Leone Spiccia 1, 2, 2, 3, 4 , Rosalie K. Hocking 3, 10, 11 , Alexandr N. Simonov 1, 2, 2, 3, 4
Affiliation
|
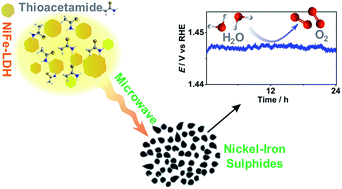
The present work introduces a rapid low-temperature microwave-assisted synthesis of nickel(iron) layered hydroxides and sulphides that exhibit robust catalytic activity for electrooxidation of alkaline water – the most feasible source of electrons for any renewable fuel synthesis. The procedures require not more than an hour to complete at 120–150 °C with quantitative yields of: (i) few-atomic-layers thick porous sheets of Ni0.75Fe0.25(OH)2+x with surface area ABET = 149 m2 g−1, and (ii) interconnected Ni0.75Fe0.25S2+y particles of few nanometers in size covered with a thin oxide/hydroxide layer having ABET = 87 m2 g−1. These and other morphological and structural features of the materials were inferred from XRD, XPS, Ni- and Fe-edge EXAFS/XANES, TEM/SAED, EDX mapping, SEM, N2 adsorption–desorption, and electrochemical techniques. At lower loadings on the electrode surface (≤0.01 mg cm−2), the specific activity for water (1 M KOH) electrooxidation at 0.3 V overpotential is 210 A g−1 for Ni0.75Fe0.25(OH)2+x, and 384 A g−1 for Ni0.75Fe0.25S2+y, which excels the performance of the best-performing analogues. The enhanced electrocatalytic activity of sulphides over hydroxides is defined by the better electrical conductivity and different nature of the electrochemically active surface species. At higher loadings, the activity of the microwave-synthesised NiFe catalysts is found to be partially limited by agglomeration, though still high enough to enable the water oxidation rate of 10 mA cmgeom−2 at overpotentials of only 0.270 ± 0.005 (flat support) and 0.21 V (foam support) with Ni0.75Fe0.25S2+y. The developed methods offer a new facile strategy for the creation of high-performing multicomponent catalysts.
中文翻译:

高度分散且无序的镍铁层状氢氧化物和硫化物:坚固且高活性的水氧化催化剂†
本工作介绍了一种快速低温微波辅助合成的镍(铁)层状氢氧化物和硫化物,对碱性水的电氧化显示出强大的催化活性,碱性水是任何可再生燃料合成中最可行的电子来源。该程序在120–150°C下需要不超过一个小时才能完成,并具有定量的产量:(i)原子层厚度仅为0.75 0.25的Ni 0.75 Fe 0.25(OH)2+ x的几个原子层厚的多孔片材,表面积A BET = 149 m 2 g -1,和(ii)相互连接的Ni 0.75 Fe 0.25 S 2+ y在大小几纳米的颗粒覆盖有具有薄氧化物/氢氧化物层甲BET =87米2克-1。从XRD,XPS,Ni和Fe边缘EXAFS / XANES,TEM / SAED,EDX作图,SEM,N 2吸附-脱附和电化学技术可以推断出材料的这些及其他形态和结构特征。在电极表面上的较低荷重(≤0.01mg cm -2)下,对于Ni(0.75 Fe 0.25(OH)2+ x),在0.3 V过电势下,水(1 M KOH)电氧化的比活为210 A g -1,并且Ni 0.75 Fe的384 A g -10.25 S 2+ y,它具有最佳表现的同类产品的性能。硫化物对氢氧化物的增强的电催化活性由更好的电导率和电化学活性表面物质的不同性质决定。在较高的负载量下,发现微波合成的NiFe催化剂的活性受到团聚的限制,尽管仍然足够高,以至于在仅0.270±0.005的超电势下,水氧化速率为10 mA cm geom -2(平坦的载体) Ni 0.75 Fe 0.25 S 2+ y和0.21 V(泡沫支撑)。所开发的方法为创建高性能多组分催化剂提供了一种新的简便策略。
更新日期:2018-04-25
中文翻译:

高度分散且无序的镍铁层状氢氧化物和硫化物:坚固且高活性的水氧化催化剂†
本工作介绍了一种快速低温微波辅助合成的镍(铁)层状氢氧化物和硫化物,对碱性水的电氧化显示出强大的催化活性,碱性水是任何可再生燃料合成中最可行的电子来源。该程序在120–150°C下需要不超过一个小时才能完成,并具有定量的产量:(i)原子层厚度仅为0.75 0.25的Ni 0.75 Fe 0.25(OH)2+ x的几个原子层厚的多孔片材,表面积A BET = 149 m 2 g -1,和(ii)相互连接的Ni 0.75 Fe 0.25 S 2+ y在大小几纳米的颗粒覆盖有具有薄氧化物/氢氧化物层甲BET =87米2克-1。从XRD,XPS,Ni和Fe边缘EXAFS / XANES,TEM / SAED,EDX作图,SEM,N 2吸附-脱附和电化学技术可以推断出材料的这些及其他形态和结构特征。在电极表面上的较低荷重(≤0.01mg cm -2)下,对于Ni(0.75 Fe 0.25(OH)2+ x),在0.3 V过电势下,水(1 M KOH)电氧化的比活为210 A g -1,并且Ni 0.75 Fe的384 A g -10.25 S 2+ y,它具有最佳表现的同类产品的性能。硫化物对氢氧化物的增强的电催化活性由更好的电导率和电化学活性表面物质的不同性质决定。在较高的负载量下,发现微波合成的NiFe催化剂的活性受到团聚的限制,尽管仍然足够高,以至于在仅0.270±0.005的超电势下,水氧化速率为10 mA cm geom -2(平坦的载体) Ni 0.75 Fe 0.25 S 2+ y和0.21 V(泡沫支撑)。所开发的方法为创建高性能多组分催化剂提供了一种新的简便策略。



























 京公网安备 11010802027423号
京公网安备 11010802027423号