当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Highly Selective and Potent Ectonucleotide Pyrophosphatase-1 (NPP1) Inhibitors Based on Uridine 5′-Pα,α-Dithiophosphate Analogues
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2018-04-22 00:00:00 , DOI: 10.1021/acs.jmedchem.7b01906 Vadim Zelikman 1 , Julie Pelletier 2 , Luba Simhaev 1 , Aviad Sela 1 , Fernand-Pierre Gendron 3 , Guillaume Arguin 3 , Hanoch Senderowitz 1 , Jean Sévigny 2, 4 , Bilha Fischer 1
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2018-04-22 00:00:00 , DOI: 10.1021/acs.jmedchem.7b01906 Vadim Zelikman 1 , Julie Pelletier 2 , Luba Simhaev 1 , Aviad Sela 1 , Fernand-Pierre Gendron 3 , Guillaume Arguin 3 , Hanoch Senderowitz 1 , Jean Sévigny 2, 4 , Bilha Fischer 1
Affiliation
|
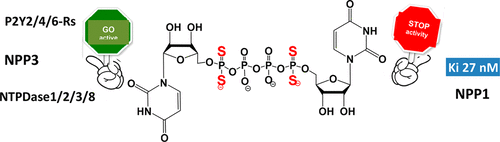
Ectonucleotide pyrophosphatase/phosphodiesterase-1 (NPP1) hydrolyzes phosphodiester bonds of nucleotides such as ATP, resulting mainly in the formation of AMP and pyrophosphate. NPP1 activity plays a deleterious function in calcified aortic valve disease and calcium pyrophosphate deposition disease. Thus, inhibitors of NPP1 represent a medical need. We developed novel NPP1 inhibitors based on uridine 5′-Pα,α-dithiophosphate analogues, 9–12. All these analogues potently inhibited hNPP1 (80–100% inhibition) at 100 μM, with no, or minimal, inhibition of NPP3 and other ectonucleotidases (NTPDase1,2,3,8). These compounds showed nearly no activity at uracil-nucleotide sensitive P2Y2,4,6-receptors and thus represent highly selective NPP1 inhibitors. The most promising inhibitor was diuridine 5′-Pα,α,5″-Pα,α-tetrathiotetraphosphate, 12, exhibiting Ki of 27 nM. Analogues 9–12 proved to be highly stable to air oxidation and to acidic and basic pH. Docking simulations suggested that the enhanced NPP1 inhibitory activity and selectivity of analogue 12 could be attributed to the simultaneous occupancy of two sites (the AMP site and an alternative site) of NPP1 by this compound.
中文翻译:

基于尿苷5'- Pα ,α-二硫代磷酸酯类似物的高选择性和有效的核苷酸磷酸焦磷酸酶-1(NPP1)抑制剂
核苷酸焦磷酸酶/磷酸二酯酶-1(NPP1)水解核苷酸(如ATP)的磷酸二酯键,主要导致AMP和焦磷酸的形成。NPP1活性在钙化的主动脉瓣疾病和焦磷酸钙沉积疾病中起有害作用。因此,NPP1的抑制剂代表医学上的需要。我们开发了一种基于尿苷5'--P新颖NPP1抑制剂α,α -dithiophosphate类似物,9 - 12。所有这些类似物均以100μM的浓度有效抑制hNPP1(抑制80-100%),而对NPP3和其他胞外核苷酸酶(NTPDase1,2,3,8)没有抑制作用或抑制作用很小。这些化合物对尿嘧啶核苷酸敏感的P2Y 2,4,6几乎没有活性受体,因此代表高度选择性的NPP1抑制剂。最有希望的抑制剂是二尿苷5'-Pα ,α,5 ''-Pα ,α-四硫代四磷酸12,表现出27 nM的K i。类似物9 – 12被证明对空气氧化以及酸性和碱性pH具有高度稳定性。对接模拟表明,该化合物对NPP1的抑制活性和类似物12的选择性增强,可归因于该化合物同时占据NPP1的两个位点(AMP位点和另一个位点)。
更新日期:2018-04-22
中文翻译:

基于尿苷5'- Pα ,α-二硫代磷酸酯类似物的高选择性和有效的核苷酸磷酸焦磷酸酶-1(NPP1)抑制剂
核苷酸焦磷酸酶/磷酸二酯酶-1(NPP1)水解核苷酸(如ATP)的磷酸二酯键,主要导致AMP和焦磷酸的形成。NPP1活性在钙化的主动脉瓣疾病和焦磷酸钙沉积疾病中起有害作用。因此,NPP1的抑制剂代表医学上的需要。我们开发了一种基于尿苷5'--P新颖NPP1抑制剂α,α -dithiophosphate类似物,9 - 12。所有这些类似物均以100μM的浓度有效抑制hNPP1(抑制80-100%),而对NPP3和其他胞外核苷酸酶(NTPDase1,2,3,8)没有抑制作用或抑制作用很小。这些化合物对尿嘧啶核苷酸敏感的P2Y 2,4,6几乎没有活性受体,因此代表高度选择性的NPP1抑制剂。最有希望的抑制剂是二尿苷5'-Pα ,α,5 ''-Pα ,α-四硫代四磷酸12,表现出27 nM的K i。类似物9 – 12被证明对空气氧化以及酸性和碱性pH具有高度稳定性。对接模拟表明,该化合物对NPP1的抑制活性和类似物12的选择性增强,可归因于该化合物同时占据NPP1的两个位点(AMP位点和另一个位点)。











































 京公网安备 11010802027423号
京公网安备 11010802027423号