当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Endoplasmic Reticulum-Localized Two-Photon-Absorbing Boron Dipyrromethenes as Advanced Photosensitizers for Photodynamic Therapy
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2018-04-22 00:00:00 , DOI: 10.1021/acs.jmedchem.7b01907 Yimin Zhou 1 , Ying-Kit Cheung 2 , Chao Ma 3 , Shirui Zhao 1 , Di Gao 4 , Pui-Chi Lo 4 , Wing-Ping Fong 2 , Kam Sing Wong 3 , Dennis K. P. Ng 1
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2018-04-22 00:00:00 , DOI: 10.1021/acs.jmedchem.7b01907 Yimin Zhou 1 , Ying-Kit Cheung 2 , Chao Ma 3 , Shirui Zhao 1 , Di Gao 4 , Pui-Chi Lo 4 , Wing-Ping Fong 2 , Kam Sing Wong 3 , Dennis K. P. Ng 1
Affiliation
|
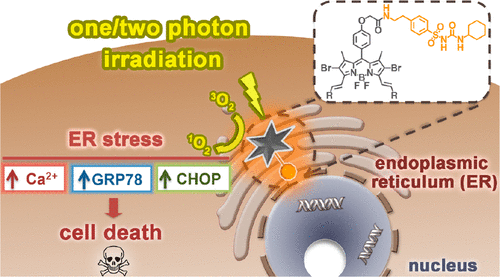
Two advanced boron dipyrromethene (BODIPY) based photosensitizers have been synthesized and characterized. With a glibenclamide analogous moiety, these compounds can localize in the endoplasmic reticulum (ER) of HeLa human cervical carcinoma cells and HepG2 human hepatocarcinoma cells. The BODIPY π skeleton is conjugated with two styryl or carbazolylethenyl groups, which can substantially red-shift the Q-band absorption and fluorescence emission and impart two-photon absorption (TPA) property to the chromophores. The TPA cross section of the carbazole-containing analogue reaches a value of 453 GM at 1010 nm. These compounds also behave as singlet oxygen generators with high photostability. Upon irradiation at λ > 610 nm, these photosensitizers cause photocytotoxicity to these two cell lines with IC50 values down to 0.09 μM, for which the cell death is triggered mainly by ER stress. The two-photon photodynamic activity of the distyryl derivative upon excitation at λ = 800 nm has also been demonstrated.
中文翻译:

内质网定位的吸收双光子的硼吡咯烷酮作为光动力疗法的先进光敏剂
两种先进的硼二吡咯亚甲基(BODIPY)基光敏剂已被合成和表征。具有格列苯脲类似物部分,这些化合物可以位于HeLa人宫颈癌细胞和HepG2人肝癌细胞的内质网(ER)中。BODIPYπ骨架与两个苯乙烯基或咔唑基乙烯基基团共轭,它们可以使Q波段吸收和荧光发射基本上发生红移,并赋予发色团两个光子吸收(TPA)特性。含咔唑类似物的TPA截面在1010 nm处达到453 GM。这些化合物还具有高光稳定性的单线态氧发生剂的作用。在λ> 610 nm处照射时,这些光敏剂对这两种具有IC 50的细胞系产生光细胞毒性值低至0.09μM,其细胞死亡主要是由内质网应激引起的。还证明了二苯乙烯基衍生物在λ= 800 nm激发时的双光子光动力学活性。
更新日期:2018-04-22
中文翻译:

内质网定位的吸收双光子的硼吡咯烷酮作为光动力疗法的先进光敏剂
两种先进的硼二吡咯亚甲基(BODIPY)基光敏剂已被合成和表征。具有格列苯脲类似物部分,这些化合物可以位于HeLa人宫颈癌细胞和HepG2人肝癌细胞的内质网(ER)中。BODIPYπ骨架与两个苯乙烯基或咔唑基乙烯基基团共轭,它们可以使Q波段吸收和荧光发射基本上发生红移,并赋予发色团两个光子吸收(TPA)特性。含咔唑类似物的TPA截面在1010 nm处达到453 GM。这些化合物还具有高光稳定性的单线态氧发生剂的作用。在λ> 610 nm处照射时,这些光敏剂对这两种具有IC 50的细胞系产生光细胞毒性值低至0.09μM,其细胞死亡主要是由内质网应激引起的。还证明了二苯乙烯基衍生物在λ= 800 nm激发时的双光子光动力学活性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号