当前位置:
X-MOL 学术
›
J. Proteome Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Characterization of Plasmodium falciparum Atypical Kinase PfPK7- Dependent Phosphoproteome.
Journal of Proteome Research ( IF 3.8 ) Pub Date : 2018-04-30 , DOI: 10.1021/acs.jproteome.8b00062 Brittany N Pease 1 , Edward L Huttlin 2 , Mark P Jedrychowski 2 , Dominique Dorin-Semblat 3 , Daniela Sebastiani 1 , Daniel T Segarra 1 , Bracken F Roberts 1 , Ratna Chakrabarti 1 , Christian Doerig 4 , Steven P Gygi 2 , Debopam Chakrabarti 1
Journal of Proteome Research ( IF 3.8 ) Pub Date : 2018-04-30 , DOI: 10.1021/acs.jproteome.8b00062 Brittany N Pease 1 , Edward L Huttlin 2 , Mark P Jedrychowski 2 , Dominique Dorin-Semblat 3 , Daniela Sebastiani 1 , Daniel T Segarra 1 , Bracken F Roberts 1 , Ratna Chakrabarti 1 , Christian Doerig 4 , Steven P Gygi 2 , Debopam Chakrabarti 1
Affiliation
|
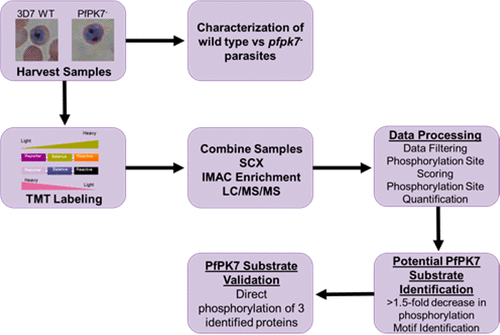
PfPK7 is an "orphan" kinase displaying regions of homology to multiple protein kinase families. PfPK7 functions in regulating parasite proliferation/development as evident from the phenotype analysis of knockout parasites. Despite this regulatory role, the functions of PfPK7 in signaling pathways are not known. To better understand PfPK7-regulated phosphorylation events, we performed isobaric tag-based quantitative comparative phosphoproteomics of the schizont and segmenter stages from wild-type and pfpk7 - parasite lines. This analysis identified 3,875 phosphorylation sites on 1,047 proteins. Among these phosphorylation events, 146 proteins with 239 phosphorylation sites displayed reduction in phosphorylation in the absence of PfPK7. Further analysis of the phosphopeptides revealed three motifs whose phosphorylation was down regulated in the pfpk7 - cell line in both schizonts and segmenters. Decreased phosphorylation following loss of PfPK7 indicates that these proteins may function as direct substrates of PfPK7. We demonstrated that PfPK7 is active toward three of these potential novel substrates; however, PfPK7 did not phosphorylate many of the other proteins, suggesting that decreased phosphorylation in these proteins is an indirect effect. Our phosphoproteomics analysis is the first study to identify direct substrates of PfPK7 and reveals potential downstream or compensatory signaling pathways.
中文翻译:

恶性疟原虫非典型激酶PfPK7依赖的磷酸化蛋白质组的表征。
PfPK7是一个“孤儿”激酶,显示与多个蛋白激酶家族同源的区域。从敲除寄生虫的表型分析可以明显看出,PfPK7在调节寄生虫的增殖/发育中起作用。尽管有这种调节作用,但PfPK7在信号传导途径中的功能尚不清楚。为了更好地了解PfPK7调控的磷酸化事件,我们对来自野生型和pfpk7-寄生虫品系的裂殖体和分段蛋白阶段进行了基于等压标记的定量比较磷酸化蛋白质组学。该分析鉴定了1,047个蛋白质上的3,875个磷酸化位点。在这些磷酸化事件中,在没有PfPK7的情况下,具有239个磷酸化位点的146种蛋白质显示出磷酸化的降低。磷酸肽的进一步分析揭示了在裂殖体和分段蛋白中pfpk7-细胞系中其磷酸化被下调的三个基序。PfPK7丢失后磷酸化水平降低表明这些蛋白质可能充当PfPK7的直接底物。我们证明了PfPK7对这些潜在的新型底物中的三种具有活性。但是,PfPK7不会使许多其他蛋白质磷酸化,这表明这些蛋白质中磷酸化水平的降低是一种间接作用。我们的磷酸化蛋白质组学分析是鉴定PfPK7的直接底物并揭示潜在的下游或补偿信号通路的第一项研究。我们证明了PfPK7对这些潜在的新型底物中的三种具有活性。但是,PfPK7不会使许多其他蛋白质磷酸化,这表明这些蛋白质中磷酸化水平的降低是一种间接作用。我们的磷酸化蛋白质组学分析是鉴定PfPK7的直接底物并揭示潜在的下游或补偿信号通路的第一项研究。我们证明了PfPK7对这些潜在的新型底物中的三种具有活性。但是,PfPK7不会使许多其他蛋白质磷酸化,这表明这些蛋白质中磷酸化水平的降低是一种间接作用。我们的磷酸化蛋白质组学分析是鉴定PfPK7的直接底物并揭示潜在的下游或补偿信号通路的第一项研究。
更新日期:2018-04-30
中文翻译:

恶性疟原虫非典型激酶PfPK7依赖的磷酸化蛋白质组的表征。
PfPK7是一个“孤儿”激酶,显示与多个蛋白激酶家族同源的区域。从敲除寄生虫的表型分析可以明显看出,PfPK7在调节寄生虫的增殖/发育中起作用。尽管有这种调节作用,但PfPK7在信号传导途径中的功能尚不清楚。为了更好地了解PfPK7调控的磷酸化事件,我们对来自野生型和pfpk7-寄生虫品系的裂殖体和分段蛋白阶段进行了基于等压标记的定量比较磷酸化蛋白质组学。该分析鉴定了1,047个蛋白质上的3,875个磷酸化位点。在这些磷酸化事件中,在没有PfPK7的情况下,具有239个磷酸化位点的146种蛋白质显示出磷酸化的降低。磷酸肽的进一步分析揭示了在裂殖体和分段蛋白中pfpk7-细胞系中其磷酸化被下调的三个基序。PfPK7丢失后磷酸化水平降低表明这些蛋白质可能充当PfPK7的直接底物。我们证明了PfPK7对这些潜在的新型底物中的三种具有活性。但是,PfPK7不会使许多其他蛋白质磷酸化,这表明这些蛋白质中磷酸化水平的降低是一种间接作用。我们的磷酸化蛋白质组学分析是鉴定PfPK7的直接底物并揭示潜在的下游或补偿信号通路的第一项研究。我们证明了PfPK7对这些潜在的新型底物中的三种具有活性。但是,PfPK7不会使许多其他蛋白质磷酸化,这表明这些蛋白质中磷酸化水平的降低是一种间接作用。我们的磷酸化蛋白质组学分析是鉴定PfPK7的直接底物并揭示潜在的下游或补偿信号通路的第一项研究。我们证明了PfPK7对这些潜在的新型底物中的三种具有活性。但是,PfPK7不会使许多其他蛋白质磷酸化,这表明这些蛋白质中磷酸化水平的降低是一种间接作用。我们的磷酸化蛋白质组学分析是鉴定PfPK7的直接底物并揭示潜在的下游或补偿信号通路的第一项研究。











































 京公网安备 11010802027423号
京公网安备 11010802027423号