当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Electrochemical Aziridination by Alkene Activation Using a Sulfamate as the Nitrogen Source
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2018-04-20 , DOI: 10.1002/anie.201801106 Jin Li 1 , Wenhao Huang 1 , Jingzhi Chen 1 , Lingfeng He 1 , Xu Cheng 1, 2, 3 , Guigen Li 1, 4
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2018-04-20 , DOI: 10.1002/anie.201801106 Jin Li 1 , Wenhao Huang 1 , Jingzhi Chen 1 , Lingfeng He 1 , Xu Cheng 1, 2, 3 , Guigen Li 1, 4
Affiliation
|
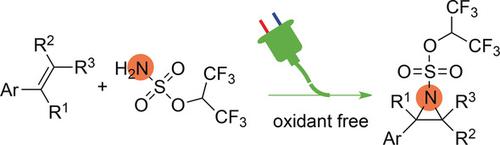
The first direct aziridination of triaryl‐substituted alkenes was achieved by means of an electrochemical process that could extend to multisubstituted styrenes. Specifically, hexafluoroisopropanol sulfamate was used as a nucleophilic nitrogen source. Mechanistic experiments suggest that this electrochemical process proceeds by stepwise formation of two C−N bonds through reactions between cationic carbon species and the sulfamate.
中文翻译:

使用氨基磺酸盐作为氮源通过烯烃活化进行电化学氮叠氮化
三芳基取代的烯烃的第一次直接叠氮化是通过电化学方法实现的,该方法可以扩展到多取代的苯乙烯。具体而言,将六氟异丙醇氨基磺酸盐用作亲核氮源。机理实验表明,该电化学过程是通过阳离子碳物质与氨基磺酸盐之间的反应逐步形成两个C-N键而进行的。
更新日期:2018-04-20
中文翻译:

使用氨基磺酸盐作为氮源通过烯烃活化进行电化学氮叠氮化
三芳基取代的烯烃的第一次直接叠氮化是通过电化学方法实现的,该方法可以扩展到多取代的苯乙烯。具体而言,将六氟异丙醇氨基磺酸盐用作亲核氮源。机理实验表明,该电化学过程是通过阳离子碳物质与氨基磺酸盐之间的反应逐步形成两个C-N键而进行的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号