Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Development of 2-arylbenzo[h]quinolone analogs as selective CYP1B1 inhibitors†
RSC Advances ( IF 3.9 ) Pub Date : 2018-04-20 00:00:00 , DOI: 10.1039/c8ra00465j Jinyun Dong 1 , Zengtao Wang 1 , Qingqing Meng 1 , Qijing Zhang 1 , Guang Huang 1 , Jiahua Cui 1 , Shaoshun Li 1
RSC Advances ( IF 3.9 ) Pub Date : 2018-04-20 00:00:00 , DOI: 10.1039/c8ra00465j Jinyun Dong 1 , Zengtao Wang 1 , Qingqing Meng 1 , Qijing Zhang 1 , Guang Huang 1 , Jiahua Cui 1 , Shaoshun Li 1
Affiliation
|
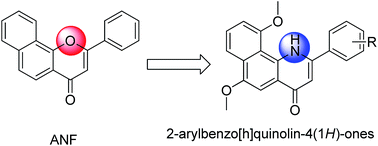
The CYP1B1 enzyme is regarded as a potential target for cancer prevention and therapy. Based on the structure of α-naphthoflavone (ANF), diverse 2-arylbenzo[h]quinolone derivatives were designed, synthesized and evaluated as selective CYP1B1 inhibitors. Compared with ANF, although few of the title compounds possessed comparable or slightly higher CYP1B1 inhibitory activity, these compounds displayed a significantly increased selectivity toward CYP1B1 over CYP1A2. Among them compounds 5e, 5g and 5h potently inhibited the activity of CYP1B1 with IC50 values of 3.6, 3.9 and 4.1 nM respectively, paralleled by an excellent selectivity profile. On the basis of predicted clog P values, these target compounds may exhibit improved water-solubility compared to ANF. In particular, 5h showed a great superiority in the reversal of CYP1B1-mediated docetaxel resistance in vitro. The current study may serve as a good starting point for the further development of more potent as well as specific CYP1B1 inhibitors capable of reversing CYP1B1-mediated anticancer-drug resistance.
中文翻译:

开发 2-芳基苯并[h]喹诺酮类似物作为选择性 CYP1B1 抑制剂†
CYP1B1酶被认为是癌症预防和治疗的潜在靶点。基于 α-萘黄酮 (ANF) 的结构,设计、合成了多种 2-芳基苯并[ h ]喹诺酮衍生物并作为选择性 CYP1B1 抑制剂进行了评估。与 ANF 相比,虽然很少有标题化合物具有相当或略高的 CYP1B1 抑制活性,但这些化合物对 CYP1B1 的选择性显着高于对 CYP1A2 的选择性。其中化合物5e、5g和5h有效抑制 CYP1B1 的活性,IC 50值分别为 3.6、3.9 和 4.1 nM,同时具有优异的选择性。基于预测的堵塞 P值,与 ANF 相比,这些目标化合物可能表现出改善的水溶性。特别是5h在体外逆转 CYP1B1 介导的多西他赛耐药方面表现出极大的优势。目前的研究可以作为一个很好的起点,进一步开发能够逆转 CYP1B1 介导的抗癌药物耐药性的更有效和特异性的 CYP1B1 抑制剂。
更新日期:2018-04-20
中文翻译:

开发 2-芳基苯并[h]喹诺酮类似物作为选择性 CYP1B1 抑制剂†
CYP1B1酶被认为是癌症预防和治疗的潜在靶点。基于 α-萘黄酮 (ANF) 的结构,设计、合成了多种 2-芳基苯并[ h ]喹诺酮衍生物并作为选择性 CYP1B1 抑制剂进行了评估。与 ANF 相比,虽然很少有标题化合物具有相当或略高的 CYP1B1 抑制活性,但这些化合物对 CYP1B1 的选择性显着高于对 CYP1A2 的选择性。其中化合物5e、5g和5h有效抑制 CYP1B1 的活性,IC 50值分别为 3.6、3.9 和 4.1 nM,同时具有优异的选择性。基于预测的堵塞 P值,与 ANF 相比,这些目标化合物可能表现出改善的水溶性。特别是5h在体外逆转 CYP1B1 介导的多西他赛耐药方面表现出极大的优势。目前的研究可以作为一个很好的起点,进一步开发能够逆转 CYP1B1 介导的抗癌药物耐药性的更有效和特异性的 CYP1B1 抑制剂。











































 京公网安备 11010802027423号
京公网安备 11010802027423号